Back | Next | Home
Missoula Technology &
Development Center
| Table
of Contents Back | Next | Home |
Missoula Technology & Development Center |
Treatment of Petroleum-Contaminated Soils
Engineers require an adequate understanding of petroleum and its movement through unsaturated soils to make decisions on the type of treatment technology for petroleum-contaminated soils. The way that petroleum was released to the soil may dictate the lateral distance that petroleum will migrate as it moves through unsaturated soil. Knowledge of the lateral extent of contamination may be a factor in choosing a treatment technology. The following brief discussion provides a description of petroleum products, a conceptual model of how they move through soil, and the climatic conditions of the Tongass and Chugach National Forests.
Soil can be contaminated with petroleum hydrocarbons from releases of crude oil or refined petroleum products such as diesel. Crude oil contains three classes of hydrocarbon compounds:
Refined petroleum also contains alkenes and alkynes, which are formed during the refining process. Examples of the structures of each of these hydrocarbon compounds are shown in figures 3 and 4.
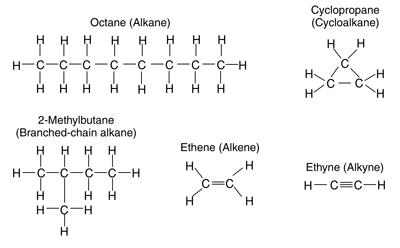
Figure 3—Typical alkanes, cycloalkane, alkenes, and alkyne.
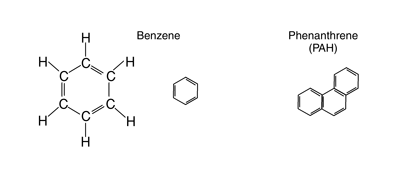
Figure 4—Typical aromatic (shown by two different schemes)
and polycyclic aromatic hydro-carbons (PAH).
Straight- and branched-chain alkanes include compounds such as propane, n-octane, and iso-octane. In these compounds, single bonds join the atoms. If a hydrocarbon only contains single bonds, the compound is considered a saturated hydrocarbon (containing the maximum number of bonded hydrogen atoms). Cycloalkanes are saturated hydrocarbons formed into a ring-type structure. This class of hydrocarbons includes such compounds as cyclopentane, cyclobutane, and methylcyclopentane.
Compounds that contain double or triple bonds (they do not contain the maximum number of bonded hydrogen atoms) are considered unsaturated. Alkenes and alkynes are unsaturated compounds. Ethene is an alkene and ethyne is an alkyne. Other alkene and alkyne compounds found in petroleum products include: 1-hexene, 2-methyl-1-butene, and so forth.
Aromatic compounds are probably more familiar to the reader. This special class of highly unsaturated hydrocarbons consists of ring-structured compounds such as benzene, toluene, ethylbenzene, and xylene (collectively known as BTEX). When carbon atoms are shared between rings, compounds are called polycyclic aromatic hydrocarbons, or PAHs.
Designing systems to reduce the mass of hydrocarbons in contaminated soils requires understanding the properties of the different classes of hydrocarbons. The key properties of hydrocarbons are:
The vapor pressure of a compound is the temperature-dependent pressure of the gas phase of the compound in equilibrium with its pure liquid phase. Vapor pressure is directly proportional to the volatility of the compound. In any particular class of hydrocarbon the vapor pressure of different compounds is going to vary by 10 to 100 times or more. Generally, the vapor pressure of most PAHs will be less than the vapor pressure of aromatics, alkanes, alkenes, and alkynes. Table 2 shows vapor pressures for some petroleum hydrocarbons of concern (Alaska Department of Environmental Conservation 2000a).
| Petroleum hydrocarbon | Vapor pressure (temperature) |
|---|---|
| 1-Butanol | 4.4 (20 °C) |
| Napthalene | 0.11 (20 °C) |
| Benzo(a)pyrene | 5.0 x 10^-11; (25 °C)* |
| Benzo(a)anthracene | 5.0 x 10^-12; (20 °C)* |
| 2-Methylphenol | 0.24 (25 °C) |
| Benzene | 76 mm (20 °C), 60 mm (15 °C) |
| Toluene | 22 mm (20 °C), 10 mm (6.4 °C) |
| Ethylbenzene | 7 mm (20 °C) |
| 0-Xylene | 5 mm (20 °C) |
| m-Xylene | 6 mm (20 °C) |
| p-Xylene | 6.5 mm (20 °C) |
| * Data from Montgomery (2000) | |
For all compounds, the vapor pressure will decrease as temperature decreases. A simple empirical equation can be used to estimate the vapor pressure of different compounds at temperatures other than the standard temperature (Schwarzenbach, Gschend, and Imboden 1993).
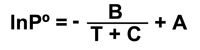
In the above equation, P° is vapor pressure (in atmospheres), T is temperature (in degrees Kelvin), and A, B, and C are constants. Values for the constants in the above equation have been tabulated for a variety of different compounds (CRC Handbook of Chemistry and Physics 1985).
Solubility measures the abundance of a compound per unit volume in the aqueous phase when the solution is in equilibrium with the pure compound at a specified pressure and temperature. Figure 5 shows the solubility of different classes of hydrocarbons as a function of the number of carbons in the compound (Curl and O'Donnell 1977). From this figure, some rules of thumb for solubility can be stated:
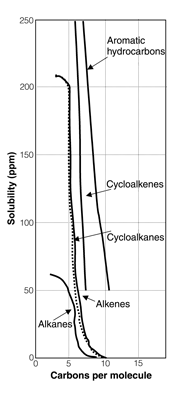
Figure 5—Solubility of the different
petroleum hydrocarbon classes
adapted from Curl and O'Donnell (1977).
Table 3 lists the solubility of some common hydrocarbons at 20 °C. The solubility of hydrocarbons will vary only by about a factor of two or less between 0 and 35 °C.
| Petroleum hydrocarbon | Solubility (mg/L) |
|---|---|
| 1-Butanol | 77,000 |
| Napthalene | 30 |
| Benzo(a)pyrene | 0.003 |
| Benzo(a)anthracene | 0.01 |
| 2-Methylphenol | 26,000* |
| Benzene | 1,780 |
| Toluene | 470 |
| Ethylbenzene | 152 |
| 0-Xylene | 175 |
| m-Xylene | 130 |
| p-Xylene | 198 |
| * Data from Montgomery (2000) | |
The volatility of hydrocarbons that are dissolved in water is quantified by a factor called the Henry's Law constant. Henry's Law is simply the ratio of the equilibrium concentration of a compound in air to the concentration of the compound dissolved in water. For treatment of petroleum-contaminated soil, this factor helps determine how effectively a hydrocarbon compound can be removed from soil and water by volatilization.
The Henry's Law constants for most hydrocarbons are temperature dependent. Calculating Henry's Law constants at different temperatures given the constant at standard temperature requires a better understanding of physical chemistry than can be presented in this manuscript. For more detailed information, refer to Schwarzenbach and others (1993) and Sawyer and others (1994). Some general rules for Henry's Law constants are:
Table 4 lists the Henry's Law constants for several common hydrocarbons. Reported values of Henry's Law constants vary over a fairly wide range for any particular compound. The values listed here are representative.
| Petroleum hydrocarbon | KH (temperature) |
|---|---|
| 1-Butanol | 7.96 x 10^-6 (25 °C) |
| Napthalene | 4.76 x 10^-4 (25 °C) |
| Benzo(a)pyrene | 3.36 x 10^-7 (25 °C) |
| Benzo(a)anthracene | 8.00 x 10^-6 (25 °C) |
| 2-Methylphenol | 1.20 x 10^-6 (25 °C) |
| Benzene | 4.76 x 10^-3 (25 °C), 3.30 x 10^-3 (10 °C) |
| Toluene | 6.70 x 10^-3 (25 °C), 3.80 x 10^-3 (10 °C) |
| Ethylbenzene | 6.60 x 10^-3 (25 °C), 3.26 x 10^-3 (10 °C) |
| 0-Xylene | 5.00 x 10^-3 (25 °C), 2.85 x 10^-3 (10 °C) |
| m-Xylene | 7.00 x 10^-3 (25 °C), 4.11 x 10^-3 (10 °C) |
| p-Xylene | 7.10 x 10^-3 (25 °C), 4.20 x 10^-3 (10 °C) |
The last property that needs to be addressed is sorption, or the partitioning of a hydrocarbon between the aqueous phase and the solid phase. Sorption of hydrocarbons onto soil particle surfaces is highly dependent on the amount of natural organic material in the soil. Different soil types will contain different amounts of natural organic material. For instance, clay contains a higher amount of natural organic material than sand. The dependence of sorption on the amount of natural organic material in soil is due to the highly hydrophobic (water-hating) nature of hydrocarbon compounds. These compounds prefer to reside on the natural organic material when they are dissolved in water.
Sorption of organic compounds is quantified as a ratio between the equilibrium concentration of the compound contained on the solid to the concentration of the compound dissolved in water. The relationship between the organic content in the soil and the sorption coefficient for organic compounds is quantified using the following equation:
![]()
where Koc is the organic carbon partitioning coefficient and foc is the fraction of organic carbon contained in the soil. Table 5 lists the Koc value for several common hydrocarbons (Montgomery 2000). Generally speaking, PAHs will have high values of Koc in comparison to other petroleum hydrocarbon classes. Unlike Henry's Law constant and vapor pressure, sorption is not highly temperature dependent (Schwarzenbach, Gschend, and Imboden 1993). As with Henry's Law constants, reported values of Koc vary over a fairly wide range. The values listed in table 5 are representative values.
| Petroleum hydrocarbon | Koc (liters per kilogram) |
|---|---|
| 1-Butanol | 3.16 |
| Napthalene | 1,300 |
| Benzo(a)pyrene | 1.15 x 10^6 |
| Benzo(a)anthracene | 3.10 x 10^5 |
| 2-Methylphenol | 50 |
| Benzene | 80 |
| Toluene | 100 |
| Ethylbenzene | 150 |
| 0-Xylene | 250 |
| m-Xylene | 170 |
| p-Xylene | 250 |
The most toxic hydrocarbons in crude oil and refined petroleum products are the aromatics. Other hydrocarbons, ordered from most toxic to least toxic, include: alkenes, cyclic alkanes, and alkanes. The most soluble, least sorptive class of compounds, aromatics, is also the most toxic. As petroleum products age (weather), the overall toxicity of the contaminant decreases because the aromatics are more volatile than the other classes of compounds.
Within each group, the toxicity of the hydrocarbons tends to decrease with increasing molecular weight. Other general rules for toxicity include:
More detailed discussion of each reaction can be found in Schwarzenbach and others (1993) and Sawyer and others (1994).
For convenience, compounds that make up petroleum are often divided into groups by the number of carbon atoms in the compounds. The more carbon atoms, the higher the boiling point. Table 6 lists the different categories.
| Petroleum hydrocarbon | Carbon atoms | Approximate boiling point |
|---|---|---|
| Gasoline range organics | C6 to C10 | 60 °C to 170 °C |
| Diesel range organics | C10 to C25 | 170 °C to 400 °C |
| Residual range organics | C25 to C36 | 400 °C to 500 °C |
Knowing the boiling point of a petroleum compound helps when determining the appropriate method for removing the compound from contaminated soil. For example, technologies that depend on volatilizing the contaminant are most effective on compounds with boiling points lower than 300 °C.
Soil cleanup levels established by regulatory agencies usually specify a maximum allowable concentration of gasoline range organics, diesel range organics, and residual range organics that can remain in soil. These maximum levels are established with consideration of such factors as depth to groundwater, mean annual precipitation, soil type, potential receptors, and so forth. For example, under State of Alaska environmental regulations (18 AAC 75.340) an algorithm has been developed to determine the cleanup levels for gasoline range organics and diesel range organics given the depth to groundwater, the mean annual precipitation, the soil type, the location of the nearest public water system, and the volume of contaminated soil (Alaska Department of Environmental Conservation 2000a). Cleanup levels for specific compounds (18 ACC 75.340) have also been established by the State of Alaska (Alaska Department of Environmental Conservation 2000a).
|
|
Back | Next Table of Contents |
Missoula Technology & Development Center |