Chapter 3—The Art and Science of Restoration
- 3.2 Making Site Amendments Based on Site Evaluations
Soil treatments will improve the soil conditions so they came as close as possible to the reference condition. Prescriptions address needs for correcting soil organic matter, nutrient balance, and biota. Soil organic matter is that fraction of the soil composed of anything that once lived. Soil compaction is broken up, and missing soil horizons are rebuilt as much as feasible. Invasive species concerns also affect the types of soil treatment that might be needed.
3.2.1 Soil Organic Matter and Mulch
Mulch can form a protective layer over the soil, blocking the impact of raindrops and keeping the soil cooler and moister during the summer and warmer during the winter. Because of the large accumulation of litter in many undisturbed systems, organic horizons can be harvested from existing forest floors (by hand raking, for instance). Litter can be spread on the impacted site to speed regeneration. Litter may have to be harvested from a different location several years later to continue helping the treated site recover. For comparison, about 2,000 pounds per acre of weed-free straw (about 1 megagram per hectare) are applied for erosion control at construction sites.
Commercial composts can also be used. Regulations on compost production should keep viable weed seeds from contaminating the site, but problems may occur. For instance, the edges of compost piles may harbor weed seeds; compost can be contaminated in trucks during transport; and weeds may blow in or grow on the compost pile after it has finished actively composting.
Many commercial composts are screened to create a uniform product. While screening produces a uniform, fine material, the material is less resistant to erosion. Coarse, unscreened composts with shreds of wood around 4 to 6 inches (100 to 150 millimeters) long are more resistant to erosion. Wood chips are not as good as shreds of wood; the chips can move because they are not long enough to intermesh. Despite these problems, composts are excellent ways to build mulches and soil organic matter in general. Although nutrient release (especially nitrogen) varies initially in compost and organic amendments, they do increasingly well in subsequent seasons, even if they are a little hot (releasing nutrients too quickly) or cold (releasing nutrients too slowly) during the first year.
A thin layer of straw is a cost-effective way of providing a minimal level of erosion protection. If native grass hay can be used, the seed heads can help provide some seeding. Thicker layers (¼ to ¾ inch, or 6 to 19 millimeters) of straw help retain soil warmth and moisture but may delay seed germination in cooler climates.
3.2.2 Soil Nutrients, pH, and Salts
Nutrients available for plants are derived from a number of sources, including organic material, atmospheric cycles, and the weathering of bedrock. Soil tests are designed to extract an amount of nutrient that is proportional to the "plant available" nutrient pools in the soil that are accessed by the plant. Each general type of nutrient is extracted by a separate test. The test for each type of nutrient must be interpreted differently, often with regard for the type of geological material at the site. Soil test data must be interpreted appropriately based on the type of sampling, soil type, and test method. Furthermore, plant growth is constrained by the most limiting soil condition, so an adequate level of one nutrient is no guarantee that all other conditions are adequate.
Comprehensive soil tests cost between $25 and more than $100 per sample. They can be used to survey a site initially, or they can be used to track the effectiveness of an amendment. Often, their cost is inexpensive compared to the overall project costs.
Comparative soil testing or, in some cases, plant tissue analysis, is the only way to determine whether the soil is deficient in nutrients. Soil nutrients or plant tissue from the restoration site are compared to samples from an undamaged control site. A soil lab will recommend how to correct soil deficiencies using fertilizers or other soil amendments. Most soil labs primarily service the agricultural industry, so test results are often geared toward remedying deficiencies of agricultural nutrients. Native plants tend to have much lower nutrient requirements. The comparative test is important because it allows the restorationist to compare the difference between undisturbed soils and soils from the impacted site. Even if nutrient levels appear to be low by agricultural standards, if plants are surviving and reproducing on the reference site, the nutrient level there can be assumed adequate for the impacted site.
To locate a soil lab, consult with your agency soil scientist or call the land grant university that operates extension services in your State. These universities have soil labs and soil chemists who work directly with the public and with agencies. Specialized soil labs also can test for biological properties of soils, such as microbial respiration or the numbers of soil micro-organisms. Agricultural labs can be used for many important tests, but be sure to interpret the results with respect to wildland sites, not agricultural sites. The revegetated or native reference site provides the comparison that is the most representative for your project conditions.
Your soil scientist and soil lab can tell you how to collect the sample. Having four replicates of soil samples provides more reliable results, and can show how much variation there is in the site. Alternatively, the samples can be mixed together (composited), which saves money, but will not show areas in the site with high or low nutrient conditions. The following example shows how soils are generally sampled.
- View the whole area and divide it into parts with similar characteristics
for sampling (landform, usage, plant type). For example, you may want to
evaluate a slope in contrast to a basin or flood plain, or north- versus
south-facing slopes. For each contrasting area of the site, try to identify
a similar "revegetated
reference" area that supports acceptable cover and diversity. Three
or four replicate samples will show whether the measured values are consistent,
but a single sample composited (mixed equally) from several samples also
can
indicate a general condition.
- If surface horizons of organics, litter,
mulch, or biotic crusts are important at the site, delineate a known
area (often 9.8
or 19.6 inches [250 or 500 millimeters] square) and harvest the organic
layer. Organic litter can be measured by weight or thickness and used
in a specification
for regenerating the impacted site. The crust, if present, can be laid
aside and reapplied after disturbance. Collecting from a defined area
allows one
to specify the weight or volume of material needed for the impacted
site.
- Dig the pit to the maximum rooting depth. This allows you
to tell whether
the pit will be successful (no buried boulders) and to see the most
general aspects
of the soil before details are evaluated. Hand-excavated pits often
are about 11.8 inches (300 millimeters) square. When the pit has been dug,
clean the
walls that will be sampled. Afterward, sample the deepest horizon
first. This horizon usually has the least organic carbon, so it is important
not to contaminate
it with debris from the A horizon. Collect about 17 ounces (500 milliliters)
of material and note the relative proportion of coarse fragments
as
a
percentage of fine (smaller than 0.08 inch [2 millimeters]) soil
material. Often, an estimate
of the coarse fragments that are not put in the bag is combined with
the sieved fractions (larger than 0.08 inch [2 millimeters] and smaller
than 0.08 inch
[2 millimeters]) from lab analysis. In this way the fine soil analysis
for nutrients and water content can be prorated for soil volumes
with high rock
content.
- Next, successively sample higher horizons from the bottom
up, so that nutrient-rich higher horizons are not sloughed down on lower
horizons. Commonly, a deepest rooting horizon is sampled (often the C horizon)
and a surface horizon (often the A horizon) with maximum nutrient content
is sampled. This often works out to be samples from 0 to 3.9 inches (0
to 100 millimeters), 7.9 to 11.8 inches (200 to 300 millimeters), and 19.7
to
23.6 inches (500 to 600 millimeters).
- Dry the samples (within 24 hours
if possible) by spreading them out to air dry or in an oven overnight.
Avoid high heat (temperatures above 113 degrees Fahrenheit, 45 degrees Celsius).
- Ship the samples immediately in properly labeled sample bags or boxes.
Avoid
using generic labels, such as "Soil A," because the lab (or your
database) may have many such samples. If plant samples are collected, it
is important to know the part of plant where the samples were taken. When
comparing plants on the reference site and those on the impacted site, sample
similar plants, and similar tissues on the plants.
- Additional information
that could help you interpret sample data later on includes the previous
type of vegetation on the site, the species to be grown there, whether
the soil was hard or loose, topsoil depth, the relative amount of roots in
the
soil (no roots, few roots, many roots), the vigor of existing vegetation,
leaf color (dark green, light green, yellow or brown spots on upper
or lower leaves), and previous soil treatments. Often, an agriculturally
oriented
lab will not be able to provide a coherent interpretation
of the analysis results for a wildland site. You should be
able to interpret the results by comparing samples from the reference site
to samples from the impacted site.
- Once you have the test results, you might use amendments or fertilizers to increase nutrients to low, but adequate, thresholds. When working with native plant communities, more is not better. Be sure the interpretations are appropriate for wildland plants, not agricultural or horticultural plants. See section 3.2.3d, Evaluating Soil Nutrients and Chemical Conditions.
Subsequent tests should be done at the same lab so the analyses are consistent. For monitoring, take soil samples at the same time of year under similar moisture conditions, if possible.
A sample site assessment field form can be found in appendix E, Forms. This form can be modified to meet your needs.
3.2.3 Rebuilding Damaged Soils
Ideally, the restorationist will attempt to re-create the structure and function of the altered soil layers. The soil assessment evaluates soil function by identifying attributes or properties that have been altered, or that would impair reestablishment of the desired plant community. For each attribute that has been damaged, the restorationist identifies a method to ameliorate the damage. The two most important issues commonly limiting plant growth on drastically disturbed sites are water availability (based on slope structural stability, infiltration, and rooting volume) and long-term nitrogen release (Claassen 2002). Nutrients other than nitrogen can be added easily by fertilizer amendments, if they are needed.
In some instances, the topsoil layer is largely intact, but its function has been impaired by compaction. Thoroughly loosening the soil may reintroduce the macropores that allow water and air to circulate. This process, called scarification, is particularly helpful in making sure that germinating seedlings have enough water available during their first weeks of establishment.
Tilling usually is not appropriate, because it mixes and pulverizes the soil, destroying soil aggregates. In some soils the rotating tines may create a tillage compaction layer. If organic matter has accumulated on the site, a priority may be to keep the topsoil layer on top where it can serve its proper function rather than tilling it into deeper soil layers. Most wilderness and backcountry sites are decompacted by breaking up the soil with a shovel or garden fork without mixing the C horizon into the organic layers.
Infiltration in a compacted soil may be improved with the incorporation of organic matter. A replacement mulch layer also may be a good idea. It is not entirely clear how important it is to keep the soil layers separate. Work by Dr. David Cole in the Eagle Cap Wilderness of Oregon (Cole and Spildie 2000) produced favorable results after various soil layers were mixed. Focus on patterns of water movement through the soil profile (no abrupt soil texture differences), accumulated organic matter on the surface (leave these valuable materials in place or replace them), and soil horizons (leave finer B horizon materials at depth and minimize damage to soil aggregates in the A horizon).
It may be necessary to crumble compacted soil by hand to break up compressed plates. The soil needs to be decompacted to the depth that plant roots need to reach (check the reference site for rooting depth). For highly compacted soils, a pick may be the tool of choice. Another tool to try in backcountry settings is the U-bar digger. This U-shaped tool has long tines at the base mounted to a crosspiece, with two long handles coming up the sides (figure 3-27). The operator steps on the crosspiece, grasps the handles, and rocks the tines back and forth to penetrate the soil. This works nicely to break up moderate compaction to just over a foot deep.

Figure 3-27—The U-bar digger is used to break up compacted soil.
The tool could be broken down for transport, but it is heavy. The tines need to be sheathed for safety if the tool is transported on stock. (See chapter 5, Tools of the Trade and Other Resources, for more information about acquiring this tool, a personal favorite of the author.)
Dr. Jayne Belnap cautions that desert soils may become more compacted after scarification, making matters worse (Belnap 2003). This may occur because fragile soil aggregates disintegrate when they are tilled. According to Belnap, it remains unclear when scarification is appropriate in a desert environment, so restorationists be warned! Find out what others have done in your area. Often, damaged soils must have organic matter mixed in to keep the soil open. Shredded wood from thinning projects could be used or harvested forest floor duff could be harvested and incorporated into the top 6 to 8 inches (150 to 200 millimeters) or so of soil. Olympic National Park uses Cocobrick, a product made from coconut fiber, to increase the organic content of soils.
3.2.3b Rebuilding Missing Layers of Soil
If the topsoil layer or deeper layers of soil are missing, as will be the case for eroded trails and excavated or sloping campsites, the task is to add one layer of soil at a time, if possible, being mindful of how limited each soil material may be in the wilderness. Soil horizons usually are added back when the site is being stabilized.
Regenerating Subsoil Horizons
If part of the B horizon is missing, it is added back first. If possible, the goal is to match the original soil profile, restoring its hydrologic characteristics. Rock or gravel may be a component of the B horizon. If you add rock and gravel, mix them with soil to fill large voids and air pockets.
Otherwise, fine materials may settle deep into the rock fill, leaving surface roots exposed in the empty spaces between the rocks.
In the wilderness, subsoil may be removed carefully from slumps on trail cutbanks, from tree wells, or from other areas where the mineral soil is exposed naturally. When borrowing soil, try to do so in a manner that will be unnoticeable and will have the least impact on the environment. In some soils, coarse fragments of wood or weathered rock have significant water-holding capacity or nutrient content and they should be included (Whitney and Zabowski 2004).
Regenerating Topsoil Horizons
Topsoil is added on top of the subsoil. Topsoil is best collected in the project location, if you can do so without causing unacceptable damage. Ideally, the topsoil should be from the same plant community you are seeking to replace through restoration. That soil will have similar properties to the soil that was lost from the disturbed site, and it will have the micro-organisms needed for successful restoration as well as the seeds of native plants. If available, clumps of topsoil may retain pore structure better than sieved or mixed topsoil.
Do not place finer textured materials, such as loams, over coarser textured soils, such as sands or gravels. The finer soils will retain more water than the coarse subsoils and will not allow the water to drain deep into the profile. Roots will tend to remain shallow and the plants will be prevented from developing deeper, drought-resistant root systems. If you cannot avoid placing fine soils over coarse soils, mix portions of each horizon to eliminate a clear textural boundary between the horizons.
Often, the best sources of topsoil (and vegetation for transplanting) include soil that is salvaged during a companion project, such as trail construction or even road construction. Organisms can survive for several months in stockpiled topsoil. If the topsoil must be stored for longer than several months, a cover crop should be planted on the windrows of topsoil to maintain biological processes. Even if salvaged topsoil completely dries out during storage, it is a valuable resource for restoration.
Other sources of topsoil might include soil that collects in drain dips along trails, soil salvaged when new drain dips are constructed, soil salvaged when removing the trail berm (also a good source of transplants), tailings scavenged from rodent burrows (such as marmot burrows), or soil collected from the roots of windthrown trees. Sometimes eroded topsoil can be borrowed from depressions where it has settled, without leaving obvious scars (figure 3-28a).

Figure 3-28a—Collecting topsoil without harming wilderness
lands
is a challenge in restoration projects. Possible sources
include topsoil
skimmed
from temporary ponds or topsoil
salvaged when drain dips
are maintained along
trails nearby.
Regenerating Organic Horizons
Depending on the site's characteristics, duff and plant litter may be added as the final surface layer. The duff will provide a source of nutrients as it continues to break down. The duff and litter also help prevent erosion and compaction by deflecting raindrops.
Duff may be mixed accidentally with the soil during planting. You can avoid this possibility by adding duff as a final mulch after planting is complete. Match the duff to the plant community you are working with. For example, if the restoration site is under trees, duff could be removed carefully from nearby tree clumps. Decomposing grasses, sedges, and forbs would be a more appropriate duff material in meadows. Duff often accumulates in pockets, such as in depressions on talus slopes, under shrubs, or in areas sheltered from the wind.
3.2.3c Amending Altered or Depleted Soils
If soil testing shows that the pH or nutritional content of a soil has been altered, the soil may require treatment or amendment, not just additional material. Naturally occurring organic material may have been stripped away, requiring replacement. A number of treatments are available. The best possible option, especially for wilderness or backcountry sites, is topsoiling. Topsoiling is adding a layer of surrogate topsoil from another source. Often the layer needs to be no more than a few inches thick to provide the nutrients, micro-organisms, and soil functions required by locally adapted native plants. The organic material may be duff collected from areas nearby, minimizing the difficulty of hauling the material.
Other methods for ameliorating soil conditions include the addition of soil amendments or fertilizer. The term, "fertilizer," refers to elemental nutrients, usually in a mix, that are applied to support seedling emergence after germination and to promote root penetration, shoot growth and vigor, and flower and seed production. Nutrients also can be added by using soil nutritional supplements. Typically these are plant, animal, or mineral materials that may contribute to the long-term improvement of soil structure. Balancing these nutrient inputs for the correct plant response is difficult and differs with each nutrient (see section 3.2.3d, Evaluating Soil Nutrients and Chemical Conditions).
Additional sources of organic material include commercial compost, peat moss, or Cocobrick. Each source has advantages and disadvantages.
Compost (figure 3-28b) has long been a superior choice for home gardening and growing crops. Compost provides a good source of organic materials and nutrients that allows for a slow release of nitrogen. Compost also has many potentially beneficial soil micro-organisms.

Figure 3-28b—Compost from an urban composting facility
provided
organic material and nutrients for a restoration
research project in the Eagle
Cap Wilderness, OR.
Compost was used successfully in the Eagle Cap Wilderness in Oregon as part of a restoration research project (Cole and Spildie 2000). Treatments including scarification, planting, and soil amendments were examined separately and in combinations. A portion of the study compared plots treated with organic material to plots treated with organic material plus additional compost. A 1-inch- (25-millimeter-) layer of organic material (moistened peat moss mixed with well-decomposed organic matter that had been collected locally) was applied to both plots and mixed to a depth of 3 inches (75 millimeters). Compost was spread 1 inch (25 millimeters) thick, watered, and worked into the top 4 inches (100 millimeters) of the soil. The combination of organic material plus compost outperformed the other treatments, supporting higher seedling densities, taller seedlings, and more canopy cover.
During a similar study in Montana, Zabinski and Cole (2000) found that adding compost benefited just one species, pearly everlasting (Anaphalis margaritacea). This study found that lack of soil moisture was more of a limiting factor than soil nutrients and organic matter, despite soil tests showing changes in soil chemistry on the disturbed site. That was also the case in Juelson's study at the Enchantment Lakes Basin in Washington (2001).
The use of imported compost in wilderness introduces a dilemma—should nonnative micro-organisms be introduced to the wilderness environment, even if they are potentially beneficial? In addition, compost may introduce disease organisms and nonnative plant seeds.
If compost is used, it should be stored for 1 or 2 years in an aerobic environment such as windrows so it is thoroughly composted; if the compost is too hot (has too much nitrogen), it will withdraw nitrogen from the plants. Compost also should be certified and tested to be weed free, although weeds can still be picked up in storage or transit. Using a liquid compost product or making your own compost tea will help alleviate weed concerns, but such treatments do not add organic material to the soil.
Using organic matter collected on the site fulfills many of the same functions as compost. It provides local microbial inoculum and it may provide a source for sustained release of nitrogen. An earthy smell produced by the filamentous bacteria Actinomycetes suggests that other microbes are active as well. Inactive soils will have little or no dirt smell. Wetland soils may have the sulfurous smell of rotten eggs.
Peat moss is used primarily to improve moisture retention in wetter environments, although in drier environments it could reduce water availability compared to clay soils. Peat bogs are not a renewable resource on a human time scale and such ecosystems are at risk of being depleted. Coconut fiber products provide a more sustainable alternative and are easy to transport in their compressed form. A Cocobrick is about the size of a loaf of bread, and can be placed in a bucket of water overnight to rehydrate.
Use of Fertilizers
Fertilizers or soil amendments may be needed when the soil does not have the nutrients to establish the desired vegetation. If soils are compacted, fertilizer may make up for the reduced function of soil micro-organisms and the decreased volume of soil where roots can grow. The addition of fertilizer also will produce the maximum growth during short growing seasons (Hingston 1982).
Native plants are adapted naturally to the nutrient content of their native soils. Unless the topsoil layer is missing from a restoration site, the use of soil amendments or fertilizer is generally not necessary and could well be counterproductive. Soil nutrients are just one of many limiting factors. Often, another factor, such as soil moisture, will be the key factor limiting plant reestablishment (Chapin 1992).
Topsoiling, mulching with organic matter that will rebuild the soil, establishing nitrogen-fixing native plants, and inoculating plants all are better options than applying fertilizer. If these techniques are not an option, fertilizer will help to establish vegetative cover initially. Fertilizer generally favors nitrogen-hungry grasses, sometimes at the expense of mid- to late-seral species, reversing the pattern of natural succession (Belnap and Sharpe 1995). This is especially true if the fertilizer is one of the common, quick-release chemical formulations that are highly soluble in water.
If topsoil is completely missing, several years of ongoing maintenance fertilization may be required. The plants will continue to need fertilizer until an organic soil layer is reestablished through decomposition of dead plant and animal matter.The potential disadvantages of fertilization include:
- Burning the roots of emerging plants with
too much nitrogen
- Increasing the damage by grazing animals that are
attracted to the most palatable plants
- Retarding reestablishment of
soil mycorrhizal fungi
- Stimulating weeds at the expense of native
plants
- Creating a nutrient pulse that is rapidly leached away
- Disrupting
the function of soil micro-organisms
- Artificially supporting plants without rebuilding soil structure
Arid lands are particularly prone to weed infestations. Restorationists should maintain low soil nutrient levels that approximate the natural condition. In a bunchgrass community, Belnap and Sharpe (1995) applied sugar at the rate of 200 pounds per acre (224 kilograms per hectare) to stimulate soil micro-organisms that would tie up soil nitrogen, discouraging the establishment of grasses and weeds while benefiting shrubs.
Overfertilization can cause adjacent water bodies to become eutrophic (rich in nutrients, but possibly poor in oxygen). Surface runoff also can carry fertilizer downslope, where it may be undesirable. Over time, chemical fertilizers leave a buildup of byproducts, including salts and heavy metals that may be toxic. Such problems are unlikely at wildland sites, given the low rates of application.
Fertilization is used extensively during reclamation of strip mines, where topsoil is missing from large acreages. In other cases, fertilization is used to increase forage for grazing.
Large-scale restorations tend to use synthetic fertilizers or sewage sludge to provide missing nutrients. In addition, they sometimes incorporate vegetative matter into the soil. Chemical fertilizers tend to weigh less than organic fertilizers, reducing the problem of transporting them. If chemical fertilizers are used, a slow-release formulation is preferred, especially for projects in arid lands where excess nitrogen will stimulate weeds. Fertilizers with chelated nutrients don't leach through the soil as quickly as other fertilizers.
Organic fertilizers may be made from plant materials, animal materials, or crushed minerals. Organic fertilizers tend to release nutrients at a slow rate, but some may do so too slowly for plants. The nutrients last longer in the soil, are taken up as the plants need nutrients, and with the exception of manure or urea, are less likely to pollute nearby water sources. Soil labs generally report suggestions for using chemical fertilizers. If you are interested in using organic fertilizers, select a suitable soil lab that will work with you, such as a lab that works with organic growers.
The respective amounts of the macronutrients in fertilizers are expressed as percentages of nitrogen (N), phosphorus pentoxide (P2O5), and potassium oxide (K2O). For example, a 20-10-5 formulation is 20-percent nitrogen, 10-percent phosphorus pentoxide, and 5-percent potassium oxide. Often, the fertilizer formulation is expressed simply as N-P-K. For instance, someone may say "The N-P-K content is 16-48-0." The percentage of elemental phosphorus in phosphorus pentoxide is calculated as the percentage of phosphorus pentoxide multiplied by 0.43. The percentage of elemental potassium is calculated as the percentage of potassium oxide multiplied by 0.83 (Redente 1993).
Chemical or organic fertilizers can be used as an interim solution to make up for the lack of nutrients. An agricultural supply company can help calculate an amendment prescription if the soil scientist on your team is not accustomed to working with soil amendments. Agricultural resources are listed in chapter 5, Tools of the Trade and Other Resources. You may also ask your local county extension agent or one of the increasing number of restoration-oriented consulting companies for suggestions.
3.2.3d Evaluating Soil Nutrients and Chemical Conditions
Soil tests use various laboratory extracts to estimate the amount of a particular nutrient that would be available to plants for some estimated period of growth. There are many different soil tests with many different interpretations—don't take test results literally. Also, wildland plants tend to have lower nutrient requirements and to need the nutrients longer than agricultural plants. Unfortunately, most soil tests were developed for agricultural systems. Always ask yourself what type of test is being used for a given nutrient and what an appropriate value would be for the field site. Some indicators of appropriate values can come from the vegetated reference site or from low-input managed systems like unimproved pastures or forests.
Soil nutrient evaluation has two steps: correlation and calibration. Correlation is the process of relating the results from the soil nutrient tests to the plant's response. Tests from an area on the reference site that has adequate plant growth can help indicate when nutrient levels are sufficient. Calibration is the process of specifying the amount of amendment needed for plant growth goals. Calibration requires knowing how much amendment is needed, if any, to bring the soil test results to an acceptable level.
Work with a soil scientist to learn how much amendment is needed, or establish several small field trials with low, medium, and high amounts of amendment. The plants' responses will tell you the correct amount of amendment. As you read through the following sections on various nutrients or soil conditions, keep the two steps in mind:
- What
is the relationship between adequate plant growth and soil test results
(correlation)?
- What amount of amendment would correct the problem and how long would it be needed (calibration)?
Soil Reaction (pH)
Soil pH is a measurement of acidity or alkalinity. A pH of 7.0 is neutral. Acidity increases exponentially below pH 7.0, while alkalinity increases exponentially above pH 7.0. Most soils have a pH between 5.0 and 7.5. Natural soils may lack certain nutrients or have tendencies to be alkaline or acidic. Often the native flora is adapted to these conditions and no correction is necessary. For example, a peat bog might have a pH of about 4.0, while an alkaline flat might have a pH of about 8.5. Soil tests also will address any toxicity issues and measure the soil's cation exchange capacity (CEC), which affects how readily plants take up nutrients.
The pH level tells how acid or alkaline a soil is, but it does not tell how much amendment is needed. For example, the pH of a cranberry may be equal to that of a grapefruit, but it will take much more antacid to neutralize all the acid in a grapefruit than in a cranberry. Consider having a soil scientist conduct buffering and neutralization tests to help estimate the amount of amendment that is needed or you can conduct field trials using several levels of lime (for excessively acid situations) or sulfur (for excessively alkaline situations) amendments. These amendments may take several months, or a wet season, to work. At an abandoned mine, acid mine drainage may neutralize even large amendments of lime and the soil eventually will become more acidic. On the other hand, the small amount of acidity in litter added as a duff layer usually will be neutralized by the soil or by decomposition.
Nitrogen (N)
Nitrogen is an essential nutrient element that forms a major component of plant proteins. Proteins form enzymes that help the plant grow, produce chemicals that make the plant unpalatable, produce viable seed, and perform other vital functions. Nitrogen availability increases root growth, enhancing the absorption of phosphorus, potassium, and other nutrients (Hingston 1982).
Nitrogen in Plants
Plants that have too little nitrogen grow poorly and may be too weak to survive drought or grazing. Their leaves may turn yellow or purple and drop early.
On substrates that are very deficient in nutrients, small nitrogen amendments may improve plant health and size and increase nitrogen fixation. On the other hand, if too much nitrogen is added to soils, weeds may find it easier to invade plant communities. Large amounts of additional nitrogen may decrease the ability of nitrogen-fixing plants to form the nodules where fixation takes place.
Often, nitrogen is added at seeding, but excess nitrogen (particularly from quick-release chemical fertilizers) can burn the roots of seedlings as they germinate. Too much nitrogen can reduce root growth and can prolong the vegetative growth phase, delaying the start of flowering and seeding. In addition, too much nitrogen may increase the succulent growth that attracts grazing animals.
At many sites, no additional nitrogen is needed. If nitrogen appears to be deficient, try to keep amendments at the low end of the scale. Because plants take several seasons to complete root growth, the most beneficial scheme for regenerating wildlands plants on low-nutrient, drastically disturbed sites is to provide low levels of available nitrogen for several years until the plant community is fully reestablished.
Nitrogen in the Soil
Nitrogen is found in soils either in inorganic or organic forms. The inorganic (mineral) forms can be highly soluble and mobile. In the form of ammonia (NH3), nitrogen volatilizes readily from alkaline soils back into the atmosphere. In the form of nitrate (NO3-), nitrogen leaches readily into the ground water. In neutral to acid soils, nitrogen often is in the ammonium form (NH4+) that is held by the negative charge on the surface of mineral and organic matter. Bacteria get energy by oxidizing ammonium to nitrate, so the ammonium form may not last long in the soil.
When soil is waterlogged, bacteria can convert nitrate to N2 gas (the original atmospheric form), in which case the fertilizer or mineralized nitrogen is lost from the "plant available" pool. Regenerating soil nitrogen pools on disturbed sites involves preventing nitrogen from being lost and re-creating some approximation of the nitrogen cycles that existed on the site before disturbance.
Typically, wildland systems have very large pools of humified soil organic matter, of which only 1 to 2 percent are decomposed (mineralized) each year. The amount of nitrogen in inorganic (mineral) forms at any one time is small.
For example, the oak/annual grass savanna of northern California has 2,605 pounds of total nitrogen per acre (2,920 kilograms of total nitrogen per hectare), of which 51 pounds per acre (57 kilograms per hectare) is mineralized each year through decomposition (Jackson and others 1988). Only 2.05 pounds of nitrogen per acre (2.3 kilograms of nitrogen per hectare) is in the inorganic forms (ammonium and nitrate) that are extracted by standard soil analysis tests.
In the Lake Tahoe Basin white fir forest, an average of 1,095 pounds of total nitrogen per acre (1,228 kilograms of total nitrogen per hectare) accumulated in the soil organic matter, with 23 pounds of nitrogen per acre (26 kilograms of nitrogen per hectare) being released by mineralization each year (Claassen and Hogan 2002). Less than 4.5 pounds of nitrogen per acre (5 kilograms of nitrogen per hectare) were extracted by standard soil analysis tests.
Many other examples of organic matter pools in wildland soils are reviewed in Claassen and Hogan (1998). The pool of total nitrogen is in the low thousands of pounds of nitrogen per acre (or kilograms of nitrogen per hectare), while the mineralizable form that is available for plant growth is about 1 to 2 percent of the total nitrogen—tens of pounds of nitrogen per acre (or kilograms of nitrogen per hectare). The small, variable amount of inorganic nitrogen (ammonium or nitrate) has little bearing on predicting plant growth, except on undisturbed sites.
Sources of Nitrogen for Soil Amendments
The most foolproof way to regenerate soil nitrogen pools is to bring in topsoil that similar in quality and quantity to the topsoil that had been on the site originally. Using topsoil provides a sustainable long-term source of nitrogen complete with soil micro-organisms (Redente 1993), seeds, and possibly plant rhizomes. The nitrogen pools in topsoil are large enough to allow plant growth to regenerate after repeated disturbances such as grazing, fire, or plant disease.
However, if just 1 percent of the total nitrogen is being mineralized each year, several thousand pounds of nitrogen will be needed per acre (or kilograms of total nitrogen per hectare), requiring applications of about 8 to 12 inches (200 to 300 millimeters) of topsoil, depending on the amount of organic matter in the topsoil. It may be cost prohibitive or logistically infeasible to provide this much topsoil.
An alternative, less intensive approach would be to provide the nitrogen needed for the plant community to regrow. The plant community will need several years to fully establish, meaning that nutrients need to be available for 2 or 3 years after amendment. Most chemical fertilizers are very soluble, ensuring that the crop or lawn gets quick access to the complete dose of nutrients. If standard chemical fertilizers were used for wildland restoration, much of the fertility intended for the second and third year of plant growth would leach away or be used to grow weeds.
Chemical coatings can slow the release of nitrogen. Fertilizer prills with polyurethane coatings are more likely to survive application by hydroseeding than sulfur- or resin-coated prills. Once weaker coatings break apart, the fertilizer is released just as fast as it would have been without the coating.
Synthetic nitrogen fertilizers come in the form of urea and ammonium nitrate, both derived from petroleum products. Urea is less expensive. Its disadvantage is that it is converted to nitrogen gas rapidly, leaches quickly, and is toxic to some plants. To compensate for the amount lost as gas, twice as much urea must be applied as would be indicated otherwise, and it should be applied on a cool, humid day. Ammonium nitrate is taken up more readily by plants, and is more effective in cold or very wet soils (Hingston 1982; Potash and Aubry 1997).
Higher availability may be more important in cold or very wet soils (Hingston 1982; Potash and Aubry 1997). Even the "slow release" chemical fertilizer formulations release nitrogen more quickly than does organic matter in topsoil.
The ratio of decomposable carbon to decomposable nitrogen determines the rate of nitrogen release from organic materials. As microbes decompose carbon in organic matter, their populations grow and they need to incorporate nitrogen to build their biomass. If the ratio of carbon to nitrogen is about 20, microbial populations grow smoothly. But when the carbon-to-nitrogen ratio is higher than 25, microbes take up the majority of the available nitrogen, a process called immobilization. Because plants are less efficient at taking up nitrogen than microbes, plants will be left with access to too little nitrogen.
| Materials | C:N Ratio |
|---|---|
| Alfalfa hay | 13:1 |
| Seaweed | 19:1 |
| Rotten manure | 20:1 |
| Apple pomace* | 21:1 |
| Leaves | 40-80:1 |
| Oat straw | 24:1 |
| Wheat straw | 80:1 |
| Paper | 170:1 |
| Decayed hemlock | 200:1 |
| Sawdust | 400:1 |
| Fresh hemlock | 500:1 |
| *Pulp after juice has been squeezed | |
Eventually, microbes use up the carbon source. As the microbes decline, more nitrogen becomes available. In contrast, organic amendments with a low carbon-to-nitrogen ratio can support microbial growth and release some of the extra nitrogen, a process called mineralization.
Woody materials may have a carbon-to-nitrogen ratio of 100 or more, so they tend to immobilize nitrogen in the surrounding area. A dead insect or rodent, on the other hand, has a carbon-to-nitrogen ratio of 5 to 10, so as the carbon decomposes, abundant nitrogen is available to be released, or mineralized.
Organic soil amendments have a range of release rates (Claassen and Carey 2004) depending on their carbon-to-nitrogen ratios and on their stage of decomposition. Some organic materials release nitrogen as quickly as chemical fertilizers and others barely release nitrogen. Several agricultural byproducts are available that release a third to half of the total nitrogen content initially, then release the rest more slowly. For instance, a product called Biosol is made from the remains of microbes used to produce pharmaceuticals. It probably decomposes in much the same way that dead microbes decompose in the soil.
Sources of nitrogen-rich organic amendments include compost and well-composted manure, but take care to find a quality source. Clopyralid, an ingredient of commercial herbicides such as Transline, Stinger, and Reclaim, can be present in compost and manure for a year-even longer under anaerobic conditions. Be wary of sewage sludge, which can have toxins (including heavy metals), and of cottonseed meal, which can have pesticide residues. Less toxic sources of nitrogen include blood meal, canola meal, and some types of vegetative residue.
Yard waste composts provide a long-term release of nitrogen similar to that of topsoil (Claassen and Carey 2004). The fibrous materials in the composts also improve surface infiltration of rainwater.
Biological nitrogen fixation occurs when free-living soil bacteria and algae fix atmospheric nitrogen. This mechanism can fix up to 50 pounds of nitrogen per acre (56 kilograms per hectare) per year, although a sagebrush community that has very few nitrogen-fixing plants may fix just 2 pounds of nitrogen per acre (2.24 kilograms per hectare) per year. Ceanothus in northern Oregon fixed 9.8 pounds of nitrogen per acre (11 kilograms per hectare) per year (McNabb and others 1979). Red alder fixed 50 to 300 pounds of nitrogen per acre (56 to 336 kilograms of nitrogen per hectare) per year (Miller and others 1989).
Nitrogen fixation requires a lot of energy. Plants that are struggling to survive cannot be expected to fix much nitrogen. They may even deplete nitrogen in the soil as they attempt to become established (Harvey and others 1989). On drastically disturbed sites, a small amendment of nitrogen may have to be added to get the nitrogen fixation system functioning.
Falling rain collects nitrogen compounds from the atmosphere that become available to plants as the rain percolates into the soil. Volcanoes, forest fires, and industrial emissions contribute ammonium to the atmosphere. Nitrate (NO3) is formed in the atmosphere by electrical discharge during thunderstorms, by ultraviolet light, and in coastal areas by saltwater that evaporates and suspends salts in the air (figure 3-29). For semiarid regions unaffected by air pollution, the nitrogen input from thunderstorms is estimated at 2 pounds per acre (2.24 kilograms per hectare) per year (Redente 1993; Salisbury and Ross 1978).
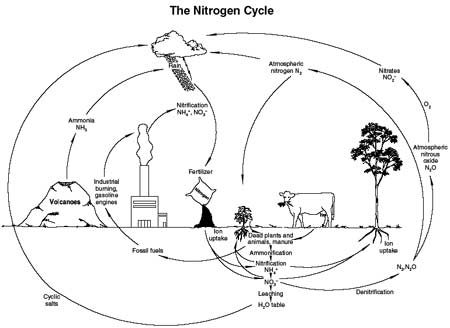
Figure 3-29—The nitrogen cycle.
In many conditions, no nitrogen amendment may be necessary. On small disturbed patches (trails, a single campsite) enough nitrogen may be available in the soil for plant growth. In areas downwind from metropolitan areas, atmospheric deposition may provide enough nitrogen. In disturbed areas that still retain some of the preexisting topsoil, tilling may allow enough nitrogen to be mineralized for the plant community to regenerate. Nevertheless, the more a site has been disturbed, the more likely that soil organic matter levels are low and that long-term supplies of nitrogen are insufficient for plant growth and community development.
The trick for appropriate amendment with fertilizers or soil amendments is to estimate the amount of nitrogen needed for each of the first two or three growing seasons. Not only do plants use nitrogen to produce roots and shoots each year, but some nitrogen will end up in plant litter, residual soil organic matter, woody stems, and the microbial community. Given the wide range of ecosystems, the amount of nitrogen any particular ecosystem requires may vary widely. When a site is restored, nitrogen amendments will be needed for each of the first several years until the various components of the plant community have regenerated.
Testing for Soil Nitrogen
Testing soils for relevant pools of plant-available soil nitrogen is difficult. Commercial soil tests are easily obtained for total nitrogen and for extractable nitrogen. The total nitrogen tests are called total elemental nitrogen analyses or if an acid digest is used, Kjeldhal nitrogen tests. These tests measure the total amount of nitrogen in the sample, including nitrogen that is not available to plants.
The extractable nitrogen tests are called potassium chloride (KCl) extracts or acetate salt extracts. These tests measure only the ammonium or nitrate that is in the soil solution being tested or that is held on the soil surfaces, a very small pool.
For an existing topsoil, nitrogen mineralization is steady enough that a quick test of extractable nitrogen will show that the process is active (1 to 10 parts per million extractable nitrogen) and that there is enough total nitrogen to keep it going for several decades. Any time the soil is disturbed or large amounts of amendment are added, it is difficult to know how much nitrogen is being produced and how long it will last.
Sites with no vegetative cover can accumulate nitrate from rainwater for a decade or so. Soil tests will detect adequate levels of nitrate and predict that corn can be grown. However, because these sites may have no organic matter (measured by total nitrogen and total carbon), the small pool of extractable nitrogen cannot be replaced once it is taken up by plants, probably within the first month of growth. Plant growth on the site would be short lived.
Until tests are available for intermediate- and long-term nitrogen availability, determining how to evaluate and amend soils depends more on empirical experience or field test plots than on laboratory tests.
Phosphorus (P)
A lack of phosphorus is the second most common soil deficiency, especially on arid lands. Phosphorus is lost through erosion of mineral and organic sediments. In contrast to nitrogen, phosphorus is immobile and insoluble in the soil. Overapplication of phosphorus is not toxic to plants, but may reduce colonization by mycorrhizae. Because of its immobility, phosphorus is not prone to leaching and must be amended into the plant's root zone (an area extending not only under the plant's canopy, but half the canopy's width beyond the canopy).
Phosphorus stimulates root growth, which is very important for seedlings. It also promotes maturity, including seed production. Phosphorus strengthens stems, helps the plants absorb nutrients, and increases disease resistance. Plants that are deficient in phosphorus may be stunted. Seedlings may have purplish foliage (Hingston 1982; Redente 1993).
Phosphorus is present in many compound fertilizers, including ammonium nitrate-phosphate (23-23-0) and ammonium phosphate (11-48-0). If only phosphorus is needed, triple superphosphate fertilizer (0-46-0) is the common choice. This formulation also contains sulfur and calcium (Hingston 1982; Redente 1993).
Organic amendments containing phosphorus include rock phosphate, basic slag, bonemeal, bloodmeal, cottonseed meal, and activated sludge. All these are applied at three to four times the rate of triple superphosphate (Rodale 1961). Because these amendments also may have significant amounts of nitrogen, be sure to balance these two elements in your amendment design. Steamed bonemeal and pelletized rock phosphate (with a lignosulphate binder) are reputed to be the best source of organic phosphorus (IFM 1995). Tilling in legumes as a green manure also will add phosphorus (Rodale 1961).
In clayey soils, the soluble phosphorus in fertilizer will eventually bind with clay particles, making it unavailable to plants temporarily. Cold soils tend to inhibit the absorption of phosphorus.
Legumes have a high phosphorus demand. Ample supplies of phosphorus will stimulate nitrogen fixation in legumes.
Soil tests are used to judge when to amend phosphorus. The extracts are generally different for acid soils than for neutral or alkaline soils, and have different minimum thresholds indicating when an amendment might be needed. Generally, extract levels less than 10 parts per million (or about 15 parts per million for acid extracts) may be low enough that a phosphorus amendment could help plants grow. Soil conditions may change the amount of phosphorus that might need to be added. Reddish soils (that are coated with oxides), clayey soils (that have a lot of surface area), or volcanic soils (that have reactive minerals) generally bind phosphorus, so they require more phosphorus when they are amended.
Composted organic matter or duff will have phosphorus in organic forms that will not fix onto surface soils as rapidly. Amendment of 110 to 220 pounds of phosphorus per acre (50 to 100 kilograms of phosphorus per hectare) in southern pine plantations has increased plant growth. In the Northwest, however, soils have higher amounts of ambient phosphorus, and their potential for fixing added phosphorus (so it can't leach through the soil) is huge. For this reason, plant response to phosphorus amendment in the Northwest is erratic and the effect of the amendment is unlikely to last for long (Powers 1989).
Potassium (K)
Potassium (K), also called potash, is taken up in large quantities by plants. It helps plants pull water from the soil, is used in many enzymes, improves seed viability, and strengthens the stem. Potassium promotes photosynthesis, root development, plant vigor, and growth, and maturation of flowers, fruits, and seeds. Potassium also increases winter hardiness in legumes (Hingston 1982; Redente 1993). Signs of potassium deficiencies in plants can include dull, bluish-green leaves with yellowing between veins, which progresses to browning leaf tips and spots or patches of discoloration. Older leaves may have rolled edges that appear to have been scorched.
Because potassium is common in minerals, it is less likely to be deficient in the soil than nitrogen or phosphorus. Its availability can be limited in coarse soils. Potassium is more mobile than phosphorus. Leaching generally is not a concern unless soils are sandy or prone to flooding. Potassium is most effective if it is incorporated into the root zone.
Potassium is taken up by waterflow into the plant's roots along with other cations (calcium, magnesium, sodium). The ratio of potassium to other cations should not be too low (less than 1 percent of the soil's cation exchange capacity), or too little potassium will be taken up. So, the extracted amount of potassium (usually around 100 parts per million or more in the soil extract) is less important than the minimum ratio of the different cations on the soil surface. In soils that are alkaline, calcium interferes with potassium absorption.
Synthetic potassium fertilizer is commonly available as potassium chloride, also called muriate of potash (0-0-60). Potassium also is available in compound fertilizers (those that include a range of nutrients).
Organic amendments used to increase potassium in the soil include kelp, manure, plant composts, granite dust, greensand, basalt rock, wood ash, and hay. Kelp is especially high in potassium and also is rich in trace elements. Leaves, canola meal, and bonemeal have less potassium (IFM 1995; Rodale 1961). Potassium is abundant but balanced in plant-based organic matter amendments such as litter and compost, so applying too much amendment is not a problem as it might be in the case of other nutrients.
Calcium (Ca)
Calcium is a component of most rock minerals, plant biomass, and lime. It may be lacking in soils that have become acidified, such as mining spoils. Calcium can be used to increase the pH of the soil, and it stimulates micro-organisms.
Calcium helps plants form cell walls and helps shoots to grow. Calcium (in the form of bonemeal) has long been used as a supplement for plants that grow from bulbs. Calcium increases the availability of phosphorus and decreases the uptake of iron, aluminum, and manganese, which can be present in toxic levels in acid soils.
The symptoms of calcium deficiencies include terminal buds that may fail to develop and leaves that may be distorted by appearing to be rolled forward along the margins or rolled backward toward the underside of the leaves. The edges of the leaves may show yellow bands or they may appear to have been scorched.
Some acid-loving species, such as blueberries and cranberries, cannot tolerate calcium.
Often calcium is amended to increase the soil's pH. Ground calcium carbonate, also called limestone flour, is a good choice if magnesium does not need to be supplemented. Calcium carbonate is one of the safer forms of calcium and is released into the soil more slowly than some other lime amendments. Ground dolomite lime (a blend of magnesium and calcium carbonates) should be used only if magnesium needs to be supplemented. If altering the soil pH is undesirable, gypsum makes an excellent calcium amendment. It contains 23 percent available calcium and 18 percent sulfur. Gypsum is a common amendment on clayey soils where it loosens the cohesion of the clay particles, making the soil more workable (IFM 1995).
Magnesium (Mg)
Magnesium occurs naturally in limestone formations. Magnesium plays a role in the formation of chlorophyll, aids in the assimilation of phosphorus, and regulates respiration. Plants with magnesium deficiencies may be discolored between the veins of older leaves or may have yellow leaves with brilliant tints (before the leaves drop). Grasses with magnesium deficiencies may be dwarfed and may have leaves with yellow stripes between the veins.
If the soil's pH does not need to be changed, magnesium sulfate is the best supplement to correct a magnesium deficiency. In acid soils, ground dolomite lime is good.
Sulfur (S)
Sulfur is fixed into the soil from the atmosphere. However, soils may still be lacking in sulfur. Soil tests for sulfur are not very accurate, so the results are approximate. The levels of sulfur on the reference site may be the best indicator of the levels needed by plants at the restoration site.
Sulfur stimulates root growth, chlorophyll production, seed production, and the formation of root nodules on legumes, which need more sulfur than grasses. Plants convert sulfur into proteins and amino acids.
Sulfur deficiencies observed in plants include leaves that turn light green, then yellow; plants that are small and spindly; and seed that matures late. In legumes, nodule formation is reduced.
Synthetic sulfur is found in compound fertilizers and is available as elemental sulfur, a byproduct of the petroleum industry. Highly basic soils are treated with the addition of elemental sulfur and certain bacteria (probably already present at the site), which form sulfuric acid, a process that may take many months.
If the soil needs to be acidified, pelletized sulfur may be used as an amendment. Otherwise, gypsum or K-mag can be used. K-mag contains 27-percent sulfur, 22-percent potassium, and 11-percent magnesium (IFM 1995).
3.2.4 Application of Fertilizers or Soil Amendments
With most restoration projects, fertilizer is added during seeding—generally in the fall. However, this timing is for convenience and cost savings. If the fertilizers are very soluble, they will burn the germinating plant roots. A better approach is to fertilize the seedlings once true leaves have appeared on them, but this requires tilling in the fertilizer and may require a separate trip to the site. With this approach, nitrogen is sure to be available when it is needed. Otherwise, it may have leached away before the seedlings need it.
Fertilizer can be broadcast with a spreader—a hand operated "belly grinder." Fertilizer should not be placed directly into planting holes unless the fertilizer is a slow-release formulation. Several formulations of "tea bag" fertilizers are easily dropped into planting holes.
Treating Saline Sites
In theory, if a soil is naturally saline, the native plants already are adapted to saline conditions. But salts can build up in the soil unnaturally because of irrigation with saline water, excessive use of fertilizers, or even because hunters or herders have placed salt blocks. If you notice a salty white crust on the soil, your site may be saline. These conditions typically occur in arid regions and in low-lying areas such as basins and playas.
Two issues arise with saline soils. The initial challenge is to discourage stock or wildlife from pawing and eating salty soil, which is not helpful when you're trying to reestablish native vegetation. In addition, if the salinity level is very high, the soil may be toxic to native plants. The best way to determine whether salinity levels may be too high is to work with a soil scientist.
Salinity can be reduced by flushing soils with copious quantities of water, or by treating soils with gypsum before flushing them. Wildlife or stock disturbance on saline soils can be reduced by reducing salinity or by using a fence or lots of logs to block access to the salty area. If human activities have hindered soil drainage, improved drainage also should be considered.
Some salt crusts are relatively mild, such as those indicated by frost-like white crusts left on pine needle litter in Yosemite National Park, CA, after rainwater pools evaporate. If the site is at high elevations or is not in an area with sedimentary rocks, salts are unlikely to be a problem.
Interpreting Soil Test Results
The soil test results in table 3-4 show comparisons of soil test results at restoration site and a revegetated reference site.
| Test | Disturbed site | Undisturbed site |
|---|---|---|
| Potassium | 52.5 ppm | 94.0 ppm |
| Potassium saturation of CEC | 1.3% | 2.0% |
| Calcium | 151 ppm | 256 ppm |
| Calcium saturation of CEC | 3.8% | 5.4% |
| Magnesium | 7.0 ppm | 24.3 ppm |
| Calcium/Magnesium ratio | 13:1 | 6:1 |
| Sodium | 26.7 ppm | 17.0 ppm |
| Sum of exchangeable bases | 1.06 meq per 3.5 oz (100 gm) | 1.79 meq per 3.5 oz (100 gm) |
| CEC | 10.0 meq per 3.5 oz (100 gm) | 11.9 meq per 3.5 oz (100 gm) |
| pH | 5.5 | 4.6 |
| Lime required for neutralization | 6.0 tons per acre (2.7 metric tons per hectare) | 15 tons per acre (6.7 metric tons per hectare) |
| Soluble salts | 1.7 millimhos per millimeter | 1.4 millimhos per millimeter |
| Phosphorus | 10.7 ppm | 18.7 ppm |
| Boron | < 0.05 ppm | 0.27 ppm |
| Organic matter | 4.8% | 9.8% |
| Estimated nitrogen release (agricultural fields) | 140 lb (63.5 kg) | 290 lb (131.5 kg) |
| Nitrate | < 0.4 ppm per 2 lb (0.91 kg) | < 0.4 ppm per 2 lb (0.91 kg) |
Soil nutrient levels should be evaluated with respect to wildland conditions. Because data on adequate nutrient levels at wildland sites are scarce, a comparison to an undisturbed reference site is valuable. Many of the soil tests are standard agricultural tests, so a large data set is available from agricultural and forestry experiments. If plants survive on your reference site, nutrient levels from that site are more meaningful than levels from agricultural fields. The pH of the soil in the Enchantment Lakes Basin is less acidic (pH 5.5) at the disturbed site than at the undisturbed site (pH 4.6). Both soils are acidic, but plants adapted to acidic soils can live there. The subsoil at both the disturbed and undisturbed sites was less acidic (pH of 5.8 at the disturbed site and 5.5 at the undisturbed site) than the soil. Because soil is more acidic on the well-vegetated undisturbed site, acidic conditions at the disturbed site probably are not the cause of poor plant growth.
The CEC (cation exchange capacity) indicates how well the soil can retain nutrient cations. It is moderate at both sites, but is 20 percent lower at the disturbed site. The proportion of nutrient (base) cations is a very low proportion of the CEC. Typically, the proportion of base cations would be more than 50 percent of the CEC. The undisturbed site also has a low proportion of base cations, but it is nearly twice that of the disturbed site. The general numerical picture is of a nutrient-stressed site. The salinity of the soil is very low, as would be expected at a high-elevation site where abundant precipitation leaches salt from the soil.
Specific nutrient analyses include potassium, calcium, magnesium, and phosphorus. Potassium occupies 1.3 (disturbed site) and 2.0 percent (undisturbed site) of the CEC (calculations not shown), which approaches values for poor agricultural soil. Calcium is very low at 3.8 and 5.4 percent of the CEC. Values over 50 percent would be expected, but the vegetated reference site is also low. One could speculate that the overlying organic layer (present on the undisturbed site but missing from the disturbed site) is a significant source of calcium for plant growth. Magnesium also was low, but was equally low on the undisturbed site. Phosphorus levels of 10.7 (disturbed site) and 18.7 parts per million (undisturbed site) are adequate for agricultural plant growth, and would not be viewed as limiting at this site.
The combination of relatively high organic matter levels, 4.8 (disturbed site) and 9.8 percent (undisturbed site), with such low calcium levels is puzzling. Interpretation of soil tests is a complex task and subject to guesswork. One technique to help interpret soil tests is to send in soils that have previously been analyzed, making sure that current results are correct or to send in multiple samples from the site. Such methods increase the cost of sample analysis.
Estimated nitrogen release is a calculated number based on nitrogen release from agricultural-quality organic matter. These values are disregarded for wildland systems except to show differences between sites. The nitrate pools change quickly with weather, plant growth, and season. Any given nitrate value has little long-term significance, except for a well-fed agricultural crop. Appropriate analysis of soil nitrogen levels is difficult for wildland situations. Given the importance of nitrogen in plant nutrition, analysis of wildland soil nitrogen levels is a major research need for revegetation science.
This brief review of soil analyses shows how soil data need to be viewed in relation to each other, in the context of the site, and with a critical eye to whether the tests were appropriate. No distinct thresholds can be defined. Plants can compensate for low levels of nutrient availability by sending their roots deeper or by greater spacing between plants.
3.2.5 Restoring Soil Biota
Depending on the degree of soil disturbance, the plant species being reestablished, and the availability of local topsoil, it may be necessary to inoculate plants with mycorrhizal fungi or nitrogen-fixing bacteria. Some species of plants cannot grow without their associated mycorrhizal fungi. Others may survive, but would not be able to grow, spread, and reproduce. Studies of soil micro-organisms have produced a fascinating, complex, and growing body of scientific information. For the purposes of restoration, the principles can be generalized to establish a fairly simple process allowing you to determine what you need for your project.
Before going into the specifics of working with mycorrhizal fungi, some background is needed. First, if vegetative cover is largely missing from a site, it is safe to assume that the soil micro-organisms have been altered or are gone altogether. Some organisms can survive in the soil for up to a few years without plant cover, but the biota will be radically different from that in areas with a full complement of plant cover. This is the case for most of our highly disturbed wilderness campsites and trails.
About 80 to 90 percent of the Earth's vascular plants are thought to depend on mycorrhizal associates. Plants that do not depend on mycorrhizal associates include the genera Saxifraga (saxifrage), Juncus (rushes), Carex (sedges), and plants in the Brassicaceae (mustard) and Caryophyllaceae (pink) families. These plants are often the first colonizers of naturally disturbed areas, such as the areas left after a glacier recedes (figure 3-30). Often, the early colonizers are replaced as the plant community develops (Cázares 1992). The lack of soil moisture or cold temperatures in arid or alpine regions limits the establishment of mycorrhizal fungi and may limit plant establishment during restoration (Amaranthus and others 1999).

Figure 3-30—As a glacier recedes, the first plants to colonize
the
raw, exposed
soils do not rely on associations with mycorrhizal
fungi.
Such plants include
the graminoids (grasses, sedges, and
rushes),
members of the mustard and pink
families, and plants
belonging to the genus Saxifraga.
Most of our weedy, introduced species do not depend on mycorrhizal associations. This characteristic is a key reason why they are able to invade disturbed areas rapidly.
In some cases, the mycorrhizal fungi needed to support young stands of a plant, such as Douglas-fir, differ markedly from those needed by mature stands. Some plants need their mycorrhizal associates all the time, while other species just call on them to help survive drought or resist disease. Sometimes the presence of one type of mycorrhizal fungus can suppress the establishment of another type, affecting survival of the plant species that depend on one species of fungus or the other (Amaranthus and others 1999).
Some species require the establishment of mycorrhizal fungi when they are young. Sometimes whole plant communities (or guilds) that follow each other in ecological succession, such as the shrub communities preceding conifer establishment, have a shared soil microflora. If the conifer species are removed, the shrub community maintains the soil flora, allowing conifers to reestablish (Amaranthus and others 1999).
The following section on the types of mycorrhizal fungi will help you determine which type of mycorrhizal fungi you might need. The most specific way to determine mycorrhizal needs is to read the scientific literature about the selected plant species.
Lacking species-specific information, experiment. To quote Aramanthus and others (1990):
The best advice for those working in reclamation [of soil organisms] is to try. One does not have to be a rocket scientist, or even have a high school diploma, in order to experiment. All it takes is common sense—perhaps backed up by a little intuition and access to the proper soil organisms.
3.2.5a Types of Mycorrhizal Fungi
Worldwide, seven general groups of mycorrhizal fungi are important to distinguish, based on the species of plants you select.
Ectomycorrhizae
This group of mycorrhizae is associated with many tree (figure 3-31) and shrub species. The prefix, ecto, refers to the fungal hyphae wrapping a web-like structure around plant roots and colonizing the spaces between the cells without penetrating the interior of the cells. Nutrients are absorbed through the cell walls of the plant's roots. Some plant roots can be linked by ectomycorrhizal hyphae, which can act like a plumbing system that allows nutrients to move from plant to plant.

Figure 3-31-Douglas-fir (Pseudotsuga menziesii) associates
with up to 2,000
different species of mycorrhizal fungi
throughout
North America (Trappe 1977).
Ectomycorrhizal fungi tend to be
generalists; one species of fungus can interact
with many different
species of plants. Drawings courtesy of the University
of
Washington
Press (Hitchcock and Cronquist 1976).
The spores of ectomycorrhizae can travel through the air and through the soil. Because the spores are so mobile, ectomycorrhizal host plants are less likely to require inoculation, even though they will still benefit.
Arbuscular Mycorrhizae
This group is associated with grasses and forbs, and with some shrub and tree species. The prefix, arbo, refers to the vesicles of the mycorrhizae, which branch in a treelike pattern. The hyphae of arbuscular mycorrhizae penetrate directly into the cells at the growing tips of plant roots. Vesicular-arbuscular mycorrhizae have large soilborne spores that migrate only short distances through the soil.
In arid and semiarid lands, 90 percent or more of the vascular plant species depend on arbuscular mycorrhizae. The arbuscular mycorrhizae fungi are less diverse than the ectomycorrhizae, but are still generalists. Sagebrush (Artemesia), for example, has only four species of fungus that interact with it (figure 3-32), but these fungi interact with other plant species as well (Moldenke and others 1994). Western redcedar (Thuja plicata), species of Ceanothus, and species of Rubus (blackberries and salmonberry) also rely on arbuscular mycorrhizae.

Figure 3-32—At least 90 percent of arid and semiarid land plants
rely on vesicular-arbuscular mycorrhizal fungi. Four such fungi
species associate
with big sage (Artemesia tridentata). Drawing
courtesy of the University of
Washington Press
(Hitchcock
and Cronquist 1976).
Ericoid Mycorrhizae
This group is specific to the heath family (Ericaceae), which includes plants such as heather, huckleberry, azalea, rhododendron, Labrador tea, and salal (figure 3-33). If you are working with a plant in the heath family, it will be important to collect inoculum from the same species.

Figure 3-33—Ericoid mycorrhizae associate with plants of the
heath
family, such as this slender wintergreen (Gaultheria ovatifolia).
Drawings
courtesy of the University of Washington Press
(Hitchcock and Cronquist 1976).
Ectendo Mycorrhizae
This group is found predominately on pines (Pinus, figure 3-34), and can occur along with ectomycorrhizae. Some plant species that are not normally thought to be mycorrhizal, such as sedges, have been colonized by ectendo mycorrhizae, particularly in alpine or arctic areas where arbuscular mycorrhizae are not well represented.

Figure 3-34—Endomycorrhizae associate with pines, such
as this
whitebark pine (Pinus albicaulis), and are more likely
to be
represented
in
subalpine or alpine environments. Drawings
courtesy of the
University of Washington
Press (Hitchcock and Cronquist 1976).
Arbutoid Mycorrhizae
This group is specific mostly to Mediterranean species, but also interacts with wintergreen (Pyrola), madrone (Arbutus, figure 3-35a) and manzanita and kinnikinnick (Arctostaphylos, figure 3-35b).
Figure 3-35a—Arbutoid mycorrhizae associate with a few
North American
genera, including genera in the heath family
such
as madrone (Arbutus).
Drawings courtesy of the
University
of Washington Press (Hitchcock and Cronquist 1976).

Figure 3-35b—Kinnikinnick (Arctostaphylos
uva-ursi) is an excellent
restoration species because it spreads readily. Drawings courtesy of
the
University
of Washington Press (Hitchcock and Cronquist 1976).
Dark Monotropoid Septate Endophytes
Not much is known about this group of mycorrhizal fungi, but it can be associated with any plant species. The dark-brown hyphae penetrate inside root cells.
Orchidaceous Mycorrhizae
As the name suggests, this type of mycorrhizal fungi is specific to the orchid family (figure 3-36). The hyphae form a spiraling structure that looks like spaghetti in the plant's root cells. While it seems unlikely that a wilderness restorationist would select an orchid species for restoration, the orchidaceous mycorrhizae are mentioned here to point out the specific needs of this beautiful and often rare group of plants.

Figure 3-36—Orchidaceous mycorrhizae associate with plants of the
orchid
family, such as this rare clustered ladyslipper (Cypripedium
fasciculatum).
Drawing courtesy of the University of Washington Press
(Hitchcock and Cronquist
1976).
3.2.5b Inoculating Plants With Mycorrhizal Fungi
While commercial cultures of some mycorrhizal fungi are available, in wilderness areas it is more appropriate to inoculate plants with mycorrhizal propagules collected near the restoration project area. Mycorrhizal propagules are spores, hyphae, and plant root fragments containing mycorrhizal fungi.
Preserving biological integrity should be a primary project goal. Too little is known about the effects of introducing new soil organisms, such as nonnative mycorrhizal fungi. They might harm the beneficial native organisms. Collecting inoculum locally also is more likely to be effective because the correct fungal species are guaranteed to be present. Some of the commercially grown inocula contain completely different species that are derived from tropical soils and may not be suitable for North American native plant communities (Cázares 2002).
Although there are many ecological uncertainties about the use of commercial inoculum in wilderness or pristine habitats, commercial inoculum could be a last resort in soil reclamation projects where mycorrhizal propagules do not exist.
The technique for collecting mycorrhizal inoculum is quite simple; dig into the root zone of the type of plant species (or group) that you will be using for restoration. Remove some soil, including small pieces of roots (figure 3-37). This soil is your inoculum. If local topsoil or organic matter is being used, the appropriate fungal spores also are likely to be present in the topsoil or organic matter.

Figure 3-37—Mycorrhizal inoculum is collected by removing soil
and
small root fragments from the root zones of healthy populations of the
same species that is being planted. The plug of vegetation dug up for this
purpose is replanted into its hole after the inoculum has been collected.
You may choose to inoculate soils when growing plants in the greenhouse or on the restoration site when planting them. Inoculating both times will enhance success. Inoculation is most successful when plants are seedlings. Inoculating a more mature plant at the restoration site after it has been grown in a nursery is less likely to succeed than if the plant is inoculated when it is being grown at the greenhouse.
If plants are being propagated offsite, use the inoculum as part of the planting medium. It is critical for the growing roots to contact fungal spores. Partially fill the flats or containers with potting soil, add a thin but continuous layer of inoculum, add the seed and cover the seed with potting soil. Mycorrhizal fungi expert Dr. Efrén Cázares, a research scientist at Oregon State University, has observed that more inoculum will survive if the inoculum is spread once the seedling plant has emerged, allowing the mutually beneficial interaction between plant and fungus to begin taking place as soon as possible. This is especially important if there is a significant lag between the time when seed is sown and germination (Cázares and others 2002).
In some instances, it may not be possible to acquire fresh inoculum just before seedling emergence. For instance, plants may be grown in the winter when the project site is under a mantle of snow. According to Cázares, inoculum can be stored in a dormant state at a cold temperature, but not frozen (Cázares and others 2005).
Plants that have been inoculated at the nursery are more likely to thrive than plants that were not. Growing conditions in the nursery will alter the soil flora because the growing conditions and nutrient levels (such as those supplied by fertilizer) are different than in the native plant community. Some nurseries may not want to inoculate seedlings because of the risk of introducing soil pathogens to the nursery. Other nurseries have ways to isolate different plant populations.
Whether plants have been inoculated at the nursery or not, they can be inoculated at the restoration site. Ideally, if you are applying topsoil, you already will be introducing mycorrhizal fungi (and soil bacteria). If it is not feasible to apply topsoil, you can spread a thin layer (about 1 to 2 teaspoons of inoculum) into the root zone of each planting hole. If you are planting seed at the restoration site, it is best to inoculate the seedling plants as soon as they emerge.
The other option is to inoculate the planting site just before seeding. The risk of this approach is that the fungi may die before the seedlings emerge. After the soil is prepared, a thin layer of inoculum is spread over the soil surface, seed is sprinkled on top of the inoculum so that the roots will push through the inoculum when they emerge, and a thin layer of soil is spread on top of the seed (depending on the species). Adding woody materials will benefit fungi by providing a reserve of moisture, as will mulch applied on top of the soil.
Depending on the environment and the plant species involved, various beneficial bacteria live in the soil and improve growing conditions for plants. The beneficial bacteria include nitrogen-fixing bacteria found in root nodules on some plants, and "free-living" aerobic bacteria found in soil crusts. Free-living bacteria do not live inside a host, as do the nitrogen-fixing bacteria that live in nodules on plant roots or the bacteria that live in the guts of insects. Some anaerobic bacteria thrive in soils saturated by water. These bacteria can be detrimental to root growth and release nitrogen from the soil into the atmosphere.
Nitrogen-Fixing Bacteria
Some types of plants host bacteria that live symbiotically in nodules on the plant's roots. These bacteria thrive by metabolizing sugars supplied by the plant, while converting atmospheric nitrogen that has washed into the soil through rain to a mineralized form that can be used by the plants. Plants that host colonies of nitrogen-fixing bacteria are called nitrogen-fixing plants. Such plants are very important to rebuilding nitrogen in damaged soils. These bacteria, as well as free-living bacteria, are likely to be present in all but the most damaged soils.
The legume or pea family (Fabaceae), the best-known group of nitrogen-fixing plants, is colonized by a bacteria called Rhizobium. Other plants (figure 3-38) host different genera of bacteria, which also may be nitrogen fixers.
Figure 3-38—Alders, once thought of as weedy tree species by foresters,
have gained more respect now that their role as nitrogen fixers is understood.
Alnus sinuata, wavy-leaved alder, has a very sweet fragrance when its
leaf
buds are opening. It is found throughout mountainous regions in the Pacific
Northwest and as far east as the Rocky Mountains of Colorado. Drawings
courtesy
of the University of Washington Press (Hitchcock and Cronquist 1976).
If a plant is a known nitrogen fixer, inoculation is similar to the method used for mycorrhizal fungi. The inoculum is collected from the same species or group of plants. However, the bacteria are found in visible nodules on the root of the plant. A slurry can be made by blending the roots with water to break open the nodules, releasing the bacteria. This slurry can be used to water nitrogen-fixing plants.
Green materials high in nitrogen (such as alfalfa crumbles) can be mixed into the soil to favor bacteria. Grasslands tend to be dominated by bacteria rather than fungi.
Some soils, especially soils lacking a litter or duff layer (such as arid or alpine soils), form visible microbiotic crusts (see figures 3-24a and 24b, and 3-25).
The restoration of soil crusts (figure 3-39) is an emerging science. Restorationists such as Dr. Jayne Belnap, who works in the arid Southwest, are leading the way. Crusts are easy to pulverize underfoot. It is best not to disturb these crusts because full recovery may take hundreds, if not thousands, of years. However, if soil crust has already been damaged (or damage is unavoidable), it may be wise to stockpile soil crust and use the stockpile to reinoculate the restoration site afterward.

Figure 3-39—Biological crusts are recognized easily by their distinct
structure. Salvaged crusts can be pulverized before being added back
to the
surface of restoration sites to inoculate the site.
Once a disturbed site in an area with soil crusts has been stabilized, backfilled, and planted, the final step is to broadcast the pulverized crust. The ideal is to replace the crust at a 1:1 ratio with the backfilled material. It is rare to have this much crust material available. A 1:10 or 1:20 ratio of crust to backfill is more common. The pulverized crust material should be no deeper than 1 inch (about 25 millimeters) after it has been spread.
If salvaged soil is to be respread immediately, the crust can be salvaged simultaneously with the topsoil layer to a depth of about 8 inches (about 200 millimeters). The live soil organisms will quickly recolonize the restoration site.
If salvaged soil must be stored before being reused, the top inch (about 25 millimeters) of crust should be removed and stored separately from the remaining 3 to 8 inches (about 80 to 200 millimeters) of topsoil. Salvaged crusts can be stored in an active or dormant state. For the crust to remain active, it must be able to photosynthesize and receive moisture. Spread the crust material just an inch or two thick. For dormant storage, store dry crust material away from moisture and sunlight, for instance, by storing it in buckets with lids. For dormant storage, the material must be dry when stored (Belnap and Furman 1997).
3.2.6 Solarization of Weedy Soils
If a restoration site is plagued by exotic plant species, it may be necessary to treat the soil before seeding or transplanting. Solarization, if done correctly, will kill most plants and seeds in the soil. Solarization, which involves covering the soil with plastic sheeting for 6 to 8 weeks, is most effective with cool-season weed and grass species. The treatment is less effective with deeply rooted summer weeds or plants that spread underground by rhizomes. Rhizomatous plants may require a second treatment, with the ground being rescarified or ripped between the treatments.
A side benefit of solarization is an increase in plant growth rates after treatment. While this effect is poorly understood, decomposition of vegetative matter may increase during solarization, making nutrients more available to plants.
However, the solarization technique kills not only weed seeds, but native seeds and some soil micro-organisms. Because the soil is nearly sterilized, solarization should be used only if the exotic species are invasive and too difficult to remove by other means. This technique also can kill pathogenic fungi, bacteria, and viruses. Interestingly, mycorrhizal fungi and earthworms seem to survive solarization.
The procedure for soil solarization follows Bainbridge (1990). Prepare the site by decompacting the soil or by spreading out salvaged topsoil 6 inches (about 150 millimeters) deep. Make sure the soil is slightly moist by adding up to 1 inch (about 25 millimeters) of water, if needed. At the time of year when weed seeds would be germinating, spread out sheets of clear 0.04- to 0.08-inch- (1- to 2-millimeter-) thick polyethylene sheeting, ideally leaving a slight airspace between the sheeting and the soil. Rocks, sticks, or small bags full of soil can be used to maintain the airspace. For windy sites, sheeting may need to be 0.16 to 0.24 inch (4 to 6 millimeters) thick. Cooler sites may need a double layer of plastic with an airspace between the layers. Bubble-pack material is inexpensive and may be worthy of experimentation. Anchor the sheeting, especially around the edges, to prevent moisture and heat from being lost and to make sure that the wind does not lift it. As an alternative, the edges of the sheeting may be buried.
Leave the sheeting in place for 6 to 8 weeks. Monitor the soil temperature (figure 3-40) using a soil thermometer. Most weed seeds are killed at temperatures of 180 degrees Fahrenheit (about 82 degrees Celsius). Some species may survive to temperatures of 212 degrees Fahrenheit (100 degrees Celsius). Any holes in the sheeting must be patched with clear packaging tape; check for holes by looking for areas where water has not condensed under the plastic. Avoid walking on the sheeting. If it is absolutely necessary to cross the sheeting, wear soft-soled shoes or go barefoot.

click on image for large and descriptive view
Figure 3-40—This thermometer shows the soil temperatures that will
kill
weed seeds, insects, and plant pathogens. Temperatures assume heat
treatment
for 30 minutes under moist soil conditions (Hartmann and others 1990).
Once the soil has been treated, minimize any further disturbance to the top 6 inches (about 150 millimeters) of the soil to prevent bringing viable weed seed deep in the soil to the surface, where it will germinate.

