| FEIS Home Page |
 |
| Figure 1—Arizona walnut growing in a desert grassland draw on the Coconino National Forest, Arizona. Creative Commons image © 2018 lunamothkd, used with permission. |
|
This review summarizes the information that was available in the scientific literature as of 2021 on the biology, ecology, and effects of fire on Arizona walnut in North America.
Arizona walnut is native to the American Southwest and Mexico. It grows primarily in riparian areas, near springs, and other areas with shallow groundwater, in mixed-deciduous, coniferous pine-oak, and oak riparian stringers, gallery forests, woodlands, and forests. Arizona walnut reproduces sexually and regenerates from seed. It also sprouts from the root crown, or higher on the trunk, after partial to complete top-kill by low- to moderate-severity fire, flood, or other disturbances. It occurs in all stages of succession, pioneering on floodplains but also growing in mid- to late-successional communities. Flooding was historically the primary disturbance in mixed-deciduous riparian communities of the Southwest; however, fire is becoming the primary disturbance in some riparian ecosystems due to changes in hydrology, climate, and plant community composition and structure. Limited research shows that Arizona walnut has relatively low postfire mortality rates, and it is most likely to survive fire as mature trees or to be top-killed and survive as sprouts. Mortality rates may increase after successive wildfires. Information on historical fire regimes in riparian areas of the Southwest is limited. Fires probably varied in severity and extent across southwestern riparian ecosystems. Most fires were likely patchy and of low severity due to the patchiness of vegetation in desert landscapes; however, mixed- and high-severity fires likely occurred as well. In many areas, stand structure and function of mixed-deciduous riparian woodlands have been significantly altered since European-American exploration and settlement began. The greatest ecological change in riparian areas since large-scale livestock ranching was introduced is probably a shift in fire regimes from mostly patchy surface fires to larger crown fires. Some research suggests that fire frequency and severity are increasing in some southwestern riparian ecosystems. More severe, intense, and/or frequent fire can interact with altered hydrology to accelerate the replacement of native plants with nonnative plants. In many riparian areas in the Southwest, there have been increases in tamarisk, Russian-olive, Mediterranean grass, and other nonnative plants that have different fuel characteristics, fire tolerances, and postfire fire responses than native species. Riparian areas are among the most diverse and productive ecosystems in desert bioregions, supporting floras and faunas that are biologically richer than adjacent uplands. Many wildlife species use riparian habits with Arizona walnut. It provides breeding, wintering, and migratory corridors for a many bird species. Riparian areas with Arizona walnut provide many ecosystem services including recreational opportunities, harboring pollinators, providing livestock forage, and enhancing water quality. Yet, many riparian communities of the Southwest are highly imperiled due to anthropogenic disturbances. Few naturally functioning riparian areas remain, and those that do are threatened with climate change. Prolonged drought may increase fire risk by decreasing water availability and reducing fuel moisture. Climate change is expected to alter water flow and lower water tables, and more frequent and severe extreme weather will likely result in more frequent and severe floods as well as more intense droughts. |
Citation:
Fryer, Janet L. 2022. Juglans major, Arizona walnut. In: Fire Effects Information System, [Online].
U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Missoula Fire Sciences Laboratory (Producer). Available: www.fs.usda.gov/database/feis/plants/tree/jugmag/all.html
[].
Juglans major var. glabrata W.E. Mannin [139]
Juglans major var. major [89,250]
Juglans major var. stewartii I.M. Johnson [241]
Arizona walnut hybridizes and intergrades with black walnut, northern California walnut, and little walnut [5,89,170]. Paradox walnut is an Arizona walnut × English walnut hybrid [170,206]. Aradhya et al. (2006) review the taxonomy and evolution of Juglans species [8].
Common names are used throughout this Species Review. For scientific names of fungi, plant, and animal species mentioned in this review and links to other FEIS Species Reviews, see the Appendix.
Reviews cited in this Species Review include: [138,148,202,233,252].
SYNONYMS
Juglans microcarpa var. major (Torr.) L.D. Benson (documented in [237])
Juglans rupestris Engelm. ex Torr. var. major Torr. [117,132]
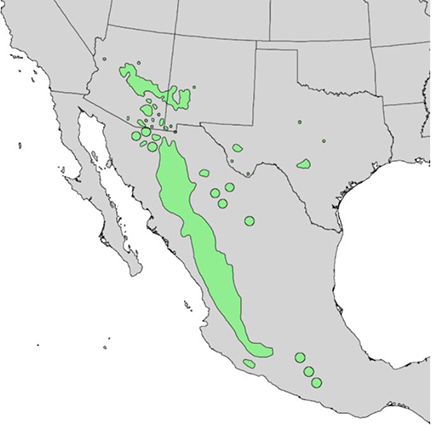 |
| Figure 2—Distribution of Arizona walnut. Map from Little (1976) [133] and digitized by Thompson et al. (1999) [232]. Arizona walnut also occurs in southwestern Utah and western Oklahoma [237]. |
Arizona walnut is native to the American Southwest and Mexico (fig. 2). It occurs from western Arizona and Oklahoma [89,114] south to southern Mexico [133]. It has established in Washington County, Utah, but it is nonnative there [254].
States:
United States: AZ, NM, OK, TX, UT [237]
Mexico: Chih, Dgo, Sin, Son [89,133,139]
SITE CHARACTERISTICS
Arizona walnut is a warm-desert riparian species that grows in areas with bimodal precipitation. Most precipitation falls in summer [184]. It is somewhat frost tolerant [99].
Arizona walnut is a phreatophyte [63,106]. It grows primarily in riparian areas, near springs, and other areas with shallow groundwater [251,252]. On the Fort Bayard Watershed of southwestern New Mexico, for example, Arizona walnut/Goodding's willow and Arizona walnut/narrowleaf willow communities occur on sites with high subsurface water or surface water over shallow bedrock [144]. Arizona walnut is most common along large streams and medium-sized rivers [251,252] but also occurs along small, higher-elevation streams and in narrow stringers along ephemeral and intermittent watercourses [66,154,181,223]. It is usually considered an obligate riparian species [20,72,88,144]. However, its water requirements are less than that of associated riparian tree species [11], and it is somewhat drought tolerant [181]. Some suggest classifying Arizona walnut as a facultative wetland species because it sometimes grows on transitional riparian-upland sites [213,215]. Ponderosa pine/Arizona walnut habitat types of the Mogollon Rim and northwestern New Mexico occur on relatively dry terraces above streams and washes [13]. Arizona walnut is flood tolerant: some root death occurs with prolonged flooding, but Arizona walnut produces adventitious roots to replace them [28].
Arizona walnut is a lowland and montane species [13,72,172] (table 1). It occurs in canyons, [50,73,188,199,241], washes [223,244], and talus [102]; and on terraces [151], foothills, and floodplains [5,24,72,73,128,132,151,154]. On the Gila River Resource Area, Arizona, Arizona walnut was most common on canyon walls or near inflows of ephemeral watercourses [151]. It is most common on north-facing slopes [226]. Riparian woodlands with Arizona walnut lie between about 1,000 and 2,130 m elevation [66,132]. In the Black Mountains of New Mexico, the importance value of Arizona walnut was highest at ≈2,000 m [91]. In 1917, Shreve reported that Arizona walnut-box elder-black cherry associations (see fig. 3) were most frequent at 1,880 m elevation [197].
| Table 1—Elevational range of Arizona walnut by area. | |
| Area | Elevational range (m) |
| Southwest | 1,012–2,195, mean of 1,595 [226] |
| Arizona | 1,100–2,100 [72,117,178] |
| New Mexico | 1,830–2,158 [2,50,91] |
Arizona walnut grows in a wide range of soil substrates [144,176]; they are often coarse, gravelly, or rocky [16,87,88,145,176]. Arizona walnut is most common in alluvium [2,3,97,180,184] and sand [2,49]. Soils supporting Arizona walnut are often stratified and may be up to 8.5 m deep on upper banks [44,144]. On the Lincoln National Forest, New Mexico, white fir/Arizona walnut riparian habitat types have rocky, sandy soils with little organic matter due to frequent flooding [2]. Parent materials on sites with Arizona walnut include basalt [195], diorite [257], granite [255], and limestone [224,256].
PLANT COMMUNITIES
Arizona walnut grows in mixed-deciduous [11,24,34,190], coniferous [2], pine-oak, and oak [119,132,145] riparian stringers [132], gallery forests, woodlands, and forests [192], and in desert and plains grasslands [159].
Mixed-deciduous
Arizona and New Mexico: Arizona walnut is most common in and dominates some mixed-deciduous riparian communities of the Southwest [34,225]. Arizona sycamore, box elder, Fremont cottonwood, netleaf hackberry, velvet ash, Goodding's willow, and other willows are common associates of Arizona walnut in these communities [11,25,30,34,88,128,150,154,165,167,175,181,192,245,246].
Arizona walnut-dominated communities (consociations) [136] occur throughout Arizona [33,165,181,225,226,227] and New Mexico [33,74,225,226,227], at around 1,439 to 2,146 m elevation [227]. Arizona walnut cover in these communities is often >70% [181]. Arizona walnut consociations are especially prominent in temporarily flooded riparian woodlands [154]. In National Forests across Arizona and New Mexico, Arizona walnut (1,768 m) and Arizona walnut-Arizona sycamore (1,458 m) communities occur at higher mean elevations than western soapberry/Arizona walnut communities (1,413 m) [181,223,226,227].
Arizona walnut frequently codominates mixed-deciduous communities of Arizona and New Mexico. It is often codominant in box elder communities [24,227]. For example, box elder-Gambel oak-Arizona walnut communities occur in Walnut Canyon National Monument, Arizona [24,227], and on banks of the Colorado River and its tributaries, Arizona walnut grows with box elder, velvet ash, and false indigo bush [55]. Western soapberry-Arizona walnut/skunkbush sumac riparian woodlands are common throughout Arizona and New Mexico, and extend into northern Chihuahua, Mexico [154,225,226]. Arizona walnut is considered characteristic of Arizona sycamore-velvet ash communities of southwestern New Mexico [225,226]. Arizona walnut, Fremont cottonwood-Arizona walnut, and velvet ash-Arizona walnut vegetation types also occur in New Mexico. California brickellbrush or western soapberry often dominate the understory [72].
Texas: Arizona walnut codominates some mixed-deciduous communities of western and central Texas. Arizona walnut woodlands occur on temporarily flooded banks and washes of the Edwards and Stockton plateaus, particularly along permanent streams [103,158]. In western Texas, Arizona walnut-desert-willow associations occur on streambanks of the Stockton Plateau [158], and chinquapin oak-Arizona walnut-slippery elm/white crownbeard forest alliances occur throughout the region [103]. Buckley's oak-Arizona walnut/Ashe's juniper and canyon maple-chinquapin oak-Arizona walnut form woodland and forest alliances on the Edwards Plateau of central Texas [159]. Black cherry, cedar-elm, and Texas live oak are frequent codominants on creek bottoms [231]. On the Balcones Canyonlands National Wildlife Refuge in central Texas, Arizona walnut codominates stream bottom riparian forests with Buckley's oak, black cherry, cedar-elm, and Ashe's juniper [230].
Conifer, conifer-oak, and oak
Arizona walnut occurs in conifer and conifer-oak woodlands and forests in riparian canyon corridors [113]. It is a component of Chihuahuan pine [3,13,181], Apache pine-Chihuahuan pine-silverleaf oak-Arizona oak, ponderosa pine-Emory oak [67,119], ponderosa pine [3,4,13,152,153,184,200], and white fir [2,4,88,153,158,184,208,246] communities. Historically, these communities often had sparse shrub understories [119]. Ponderosa pine/Arizona walnut/bunchgrass and white fir/Arizona walnut/bunchgrass associations occur on cool and cool-wet alluvial sites [184]. White fir/Arizona walnut habitat types occur the on Mogollon Rim of Arizona and New Mexico, and in southern New Mexico [2,67]. Although Arizona walnut is usually a minor type in these forests, it is considered diagnostic of that riparian habitat type [2].
Arizona walnut also occurs in juniper [69,172] and cypress [124,166] communities. In central Arizona, for example, Arizona walnut occurs in alligator juniper/catclaw acacia and alligator juniper/mule-fat woodlands [172]. In central Texas, it codominates some riparian areas with Ashe juniper and boxelder [69,70]. In the Santa Catalina Mountains of Arizona, Arizona walnut grows in Arizona cypress-Arizona alder-silverleaf oak woodlands [166]. In Kerr County, south-central Texas, bald cypress-Arizona walnut-pecan communities occur along small streams [37].
Arizona walnut is sometimes a component of encinal woodlands near riparian areas. Arizona walnut riparian woodlands may merge into drier Emory oak woodlands [181]. Arizona walnut occurs in mesic areas within Gambel oak woodlands [145], especially where those woodlands merge into riparian pine or white fir communities with Gamble oak and Arizona walnut in the understory [88]. In the Wichita Mountains of Oklahoma, Arizona walnut occurs in post oak-blackjack oak woodlands with eastern redcedar and netleaf hackberry [207].
Grasslands
Mixed-deciduous communities with Arizona walnut merge into desert (fig. 1)
or plains grasslands in lowlands, and coniferous woodlands and forests or oak woodlands in uplands. In the Southern Great Plains, Arizona walnut is considered diagnostic of riparian woodland and forest communities that merge into grasslands [159]. In New Mexico, Arizona walnut/sideoats grama communities occur in middle and upper watersheds of the Rio Grande [76].
 |
| Figure 3—An Arizona walnut-box elder-black cherry stand on the floodplain of Little Creek, a tributary of the Gila River, New Mexico. Creative Commons image by Patrick Alexander, used with permission. |
 |
| Figure 4—Staminate Arizona walnut catkins. Creative Commons image by Patrick Alexander, used with permission. |
 |
| Figure 5—Arizona walnut foliage and nuts. Image by Whitney Cranshaw, Colorado State University, Bugwood.org. Used with permission. |
BOTANICAL DESCRIPTION
This description covers characteristics that may be relevant to fire ecology and is not meant for identification. Identification keys are available (e.g., [5,73,117,171]).
Aboveground Description
Arizona walnut is a small to large tree. It is usually 9 to 15 m tall and 0.3 to 1.2 m in diameter at the base [97,117,132,241] at maturity, but it may grow up to 17 m tall [50]. It typically has a single, straight trunk [5] and a spreading upper crown [117]. Arizona walnut on the Coronado National Forest averaged 29.7 cm DBH [59]. The bark of mature trees is "thick" [132]. Leaves are pinnately compound (fig. 5) and 8 to 36 cm long [132,246]. The flowers are catkins [21]. Staminate (male, fig. 4) and pistillate (female) catkins are borne on the same tree [21,89,121]. Male catkins are 10 to 20 cm long; the smaller female catkins are 6 to 12 mm long [21]. The fruit is a hard-shelled nut [5,21,50,89,117,246] enclosed in an indehiscent, thick husk [21].
Belowground Description
Arizona walnut is deep rooted [63]. On the Walnut Gulch Experimental Watershed in southeastern Arizona, a 10-year-old Arizona walnut had a 14.3-cm diameter taproot and three major lateral roots that were 9.1, 9.0, and 6.7 cm in diameter. Smaller laterals branched off the major laterals, and there was a dense network of surface laterals ranging from 2 to 4 cm in diameter. The taproot branched in two at about 1 m belowground; length of the taproot and lateral roots was not determined [105].
Stand or Population Structure
Stand structure:
Arizona walnut-dominated communities are variable in structure [189]. They sometimes form gallery forests [136,150], but mature, mixed-deciduous stands with Arizona walnut typically form woodlands or forests [231]. The forests are often dense and have high basal areas [91]. On the eastern slope of the Black Range of New Mexico, high total basal areas (1,543-3,083 cm²/46 m²) in low-elevation (1,853 m) riparian communities were due to the presence of many large Arizona walnuts and narrowleaf cottonwoods [91]. Consociations of Arizona walnut may be shrubby or composed of short trees [181].
There are few accounts of the stand structure of mixed-deciduous communities prior to European-American settlement. When describing the Pine Springs Draw of the upper Verde River Watershed in presettlement Arizona, an expedition leader wrote that the "basin-like valley was covered with a thick growth of timber—cottonwood, walnut [Arizona], and ash [Arizona mountain-ash]" (Whipple 1854, cited in [194]). In a reassessment of vegetation along Walnut Creek, northwestern Arizona, Shaw (2006) determined that the aerial distribution of riparian woodlands has not changed greatly since 1851, although densities within many stands have increased. The reassessment was based on early photographs, diaries, and reports compared to aerial photographs taken from 1995 to 1999 [194].
Across the landscape, mixed-deciduous communities with Arizona walnut may form patches that intermix with other riparian plant communities. Four riparian patch types were intermixed along the San Pedro River in Arizona: old-growth (>50 years old) Fremont cottonwood-netleaf hackberry-Arizona walnut, mesquite, mule-fat-narrowleaf willow, and tamarisk [9].
Several publications provide quantitative descriptions of the stand (table 2a) and age class (table 2b) structure of communities in which Arizona walnut is important:
| Table 2a—Summary information from studies that measured stand structure characteristics of riparian communities with Arizona walnut. Measurements are means for Arizona walnut unless otherwise indicated. See publications for similar data on associated species. | ||
| Location | Plant community | Measurement(s) |
| Arizona and New Mexico | Fremont cottonwood-green ash | Basal area = 292 cm²/500 m² |
| Arizona and northwestern New Mexico | Arizona walnut-Arizona sycamore | Cover = 75% (range 57%-89%) |
| Arizona and northwestern New Mexico | Arizona walnut/buckthorn woodland | |
| Arizona and northwestern New Mexico | netleaf hackberry-Arizona walnut | Cover = 81% (range: 73%-88%) [226] |
| Eastern Arizona and western New Mexico | Fremont cottonwood-Goodding's willow gallery forest | |
| New Mexico | western soapberry-Arizona walnut/skunkbush sumac | |
| New Mexico, Black Mountains | Fremont cottonwood-Arizona walnut | Stand basal area: |
| New Mexico, Fort Bayard Watershed | Arizona walnut-narrowleaf willow | |
| Texas, Balcones Canyonlands National Wildlife Refuge | Buckley's oak-black cherry-Arizona walnut stream bottom forest | |
| Table 2b—Summary information from studies that measured age class structure of riparian communities with Arizona walnut. | ||
| Arizona and northwestern New Mexico | mixed-deciduous | Density: |
| Arizona, San Pedro River | Fremont cottonwood-Goodding’s willow/Arizona walnut | Density: |
Age class structure: Arizona walnut is long-lived and may reach 400 years old [79]. Mixed-deciduous communities with Arizona walnut are often uneven-aged [9,11,148,225,228], with Arizona walnut comprising part of the uneven-aged structure. In Fremont cottonwood-netleaf hackberry-Arizona walnut stands along the San Pedro River, Arizona walnut had similar densities of seedlings, saplings, and mature trees [9] (table 2b). Surveys in Walnut Canyon National Monument found that all size classes of Arizona walnut were represented, from seedlings (<1.4 m tall) to mature trees (>15 m tall). The largest individuals had a mean height of 23 m and a mean basal diameter of 103 cm. Over 80% of Arizona walnuts had at least medium vigor (>50% live crown), indicating that trees were healthy across size classes [191].
Raunkiaer Life Form
Phanerophyte [174]
SEASONAL DEVELOPMENT
Arizona walnut is winter deciduous [136,181]. It is dormant from about mid-November to mid-March, when buds break [30]; it may also go dormant during late summer drought [218]. Arizona walnut flowers in spring [21,89,121]. Catkins appear just before or shortly after the leaves emerge [21], from around mid-March [30] to May [178]. Arizona walnut flowers in April in Texas [250]. The nuts mature in about 3 months, ripening from July to September, depending on location and weather [219]. The nuts turn from green to dark brown or black, and drop, from August [30] to September [219]. Some nuts may be retained and disperse during spring floods [26]. Germination usually coincides with summer rains or less frequently, with spring rains [218].
Pollination and Breeding System
Arizona walnut is monoecious [21,217], with separate staminate and pistillate catkins [179,241]. Trees on relatively dry sites tend to produce more male flowers than trees on more mesic sites, and trees on the driest sites may produce only male flowers [217].
Seed Production
Arizona walnut may produce large seed crops every 2 to 3 years [171,241]. Arizona walnut reproduction is highly dependent on favorable precipitation and periodic floods [202]. Female flower production decreases during times of stress (e.g., drought) [217].
Masting is infrequent but may occur in years of abundant rainfall [218]. Seed production and size tend to increase with precipitation [219]. However, trees on relatively dry sites tend to produce fewer, but larger, seeds than trees on streambanks [12,219].
Seed predation reduces the seed crop. Arizona gray [58,218,219] and Chiricahua fox [120] squirrels eat some of the nuts. They generally eat them immediately and do not cache them [218].
Seed Dispersal
Most nuts fall under or near parent trees. Gravity can disperse nuts on slopes, and rodents may disperse nuts on- and off-site [9].
Seed Banking
Arizona walnut has a transient soil seed bank of nuts in litter and shallow soil layers. The nuts are short-lived due to their high lipid content [21]. In Canyon de Chelly National Monument, Arizona, Arizona walnut was present in aboveground vegetation but not in soil seed bank samples [183].
Germination
Arizona walnut nuts are dormant upon dispersal. They are
nonrefractory (Fryer 2022, this publication). Cold
stratification (i.e., overwintering) breaks dormancy [21]. The nuts germinate in their shells the spring or summer following production [29,218], usually coinciding with spring or summer rains [218]. Germination rates average about 50%, ranging from 10% to 64% [241]. Buried nuts have higher germination rates than nuts on the soil surface [218]. Seed viability tends to increase as seed size increases; however, seed weight and viability may decline as large trees senesce. Germination rates may be highest on open sites. In central Arizona, germination of Arizona walnut was lower on an open site with a dense herbaceous cover than on a site with an open canopy but sparse herb and shrub layers [219].
Seedling Establishment
Arizona walnut seedlings establish over wide light gradients [219]. Seedlings may establish under open to closed canopies (see Successional Status), although open, moist sites are most favorable. In southwestern New Mexico, high densities of Arizona walnut seedlings occurred in stands with a sparse overstory canopy [144]. However, advanced Arizona walnut regeneration occurred in the understories of late-successional Fremont cottonwood-Arizona sycamore and narrowleaf cottonwood-Arizona alder communities on the Gila River Watershed in New Mexico [154].
Abundant rainfall [218] and/or moist soils can result in high rates of Arizona walnut seedling establishment [218], while scouring, flooding [94,190], dry soils, drought [218], and/or grazing [190] reduce rates of establishment. Along a topographic gradient in a riparian forest stand along West Clear Creek in central Arizona, the absence of Arizona walnut seedlings from active channels suggests that scouring may inhibit its establishment on those sites, and the absence of seedlings under mature trees on upper benches suggests that the drier soils may inhibit establishment on those sites [94]. Seedling establishment on upper benches may occur only in years of abundant rainfall [218]. Along the San Pedro River in Arizona, most Arizona walnut juveniles occurred on sites where depth to groundwater averaged between 1 to 2 m below the floodplain [215].
Plant Growth and Mortality
Arizona walnut is relatively slow growing [50], although seedlings may grow rapidly on moist, open sites [181,241]. Seedlings allocate most growth to taproots to access moist soil layers [181]. Beyond the seedling stage, Arizona walnut averages about 0.3 m of top-growth per year [49,190]. In central Arizona, Arizona walnut had the slowest growth rate among six associated riparian species, with radial growth averaging 1.2 mm/year. Stream flow was positively associated with annual radial growth [94].
Drought is the primary cause of Arizona walnut mortality in all age classes [202], although fungi also kill Arizona walnuts. Although Arizona walnut wood resists decay [221], senescent walnut trees are typically infested with root rot fungi that eventually kill them [186].
Vegetative Reproduction and Regeneration
Arizona walnut sprouts from the root crown after top-killing disturbances such as flood or fire [19,252] (see Fire Adaptations and Plant Response to Fire). It may produce epicormic sprouts from higher on the trunk if not completely top-killed [19].
SUCCESSIONAL STATUS
Arizona walnut occurs in all stages of succession [13,218]. It may pioneer on floodplains [9,109], but it is also a shade tolerant [9,30,144,154,218], mid- to late-successional tree [11,144,220].
Arizona walnut grows in all stand strata (understory, midcanopy, and overstory). It can establish beneath an overstory canopy; advance Arizona walnut regeneration grows in both mixed-deciduous (see table 2b) and conifer communities [3,9,30,144,154]. On sites throughout southeastern Arizona and southwestern New Mexico, Arizona walnut seedlings were "fairly common" in Arizona sycamore-Arizona walnut communities [225,228]. In a mixed-deciduous community along O'Donnell Creek in southeastern Arizona, most Arizona walnut individuals were understory seedlings (0-12 cm tall, 48%) or saplings (13-23 cm tall, 25%). Only 4% were >52 cm tall [11]. On the Fort Bayard Watershed of southwestern New Mexico, Arizona walnut grew as a midstory tree in a lanceleaf cottonwood-narrowleaf willow community [144]. A mostly closed-canopy Arizona walnut-western soapberry-gum bully woodland occurs along intermittent streams at the Fort Bowie National Historic Site, Arizona [247].
Flooding is the primary disturbance in mixed-deciduous riparian communities of the Southwest [87,201,212,251]. Due to erosion and deposition during floods, these communities often experience large-scale disturbances that set back succession [45,148,251]. Early-successional stages are common in mixed-deciduous woodlands [45,251], and trees on heavily scoured sites may never reach maturity [251] (i.e., the stem exclusion stage).
Arizona walnut also establishes in early postfire environments (see Fire Adaptations and Plant Response to Fire). Fire is becoming the primary disturbance in riparian ecosystems due to changes in hydrology (see Fire Management Considerations), climate, and plant community composition and structure (i.e., fuels) [253] (see Fire Regimes).
Invasive tamarisk may alter successional pathways in riparian communities with Arizona walnut, resulting in lower stand density [216,220] and/or basal area of Arizona walnut. Along the San Pedro River in southeastern Arizona, basal area and canopy height of overstory trees, including Arizona walnut, increased with stand age in stands without saltcedar, a tamarisk species, but not in stands with saltcedar [216]. See Fire Regimes for further information on the effects of tamarisk invasion.No information was available on the effects of fire on Arizona walnut nuts. Nuts in the upper canopy may survive low-severity surface fire. The thick shell may help insulate Arizona walnut embryos from low-severity fire. Nuts buried in soil are likely protected from many fires; however, viable nuts only persist in the soil seed bank for a short time.
 |
| Figure 6—An Arizona walnut stand after the 2011 Miller Creek Wildfire on the floodplain of Little Creek, a tributary of the Gila River, New Mexico. This stand is at a lower elevation than that shown in figure 3. Creative Commons image by Patrick Alexander, used with permission. |
Postfire Regeneration Strategy
Tree with a sprouting root crown [210]
Other Possible Strategies:
Ground residual colonizer (on site, initial community)
Crown residual colonizer (on site, initial community)
Initial off-site colonizer (off site, initial community)
Secondary colonizer (on- or off-site seed sources) [210]
FIRE ADAPTATIONS AND PLANT RESPONSE TO FIRE
Arizona walnut sprouts from the root crown, or higher on the trunk, after partial to complete top-kill by low- to moderate-severity fire [19,252]. It may also establish from seed from off- [112] and on-site seed sources, although this was not documented. The nuts may disperse from on-site parents that survive low-severity surface fire. Gravity and cache-hording rodents [9] may disperse the nuts onto burns from on- or off-site. Nuts buried in soil [218] and on open sites [219] tend to have favorable germinate rates, so they may germinate and establish in postfire environments.
There are few data or observations on the response of Arizona walnut to fire. In a Buckley's oak-Ashe juniper/eastern poison-ivy-sweet mountain grape woodland on the Fort Hood Military Reservation in east-central Texas, density of walnuts (mostly Arizona walnut, but also black walnut) was zero immediately after both a 1996 and a 2009 wildfire, and walnuts remained absent for the first 1 to 2 years after each fire. Prefire density was not available. Walnut density steadily increased over time (2-14 years) after the first fire. Long-term postfire data (>2 years) were not available for the second fire [177]. Quantitative data on these increases were not provided for walnuts; however, data are available for 38 of 47 associated taxa affected by the wildfires. See the Management Project Summary of this study for more information.
In 2003–2004 and again in 2012–2013, Bock and Bock (2014) measured the condition of riparian trees at the Appleton-Whittell Research Ranch, Arizona, in three canyons above the San Pedro River that were burned by wildfire in 2002, 2009, or both. Fire damage to Arizona walnut was intermediate compared to four other deciduous tree species studied. Fire killed some Arizona walnuts, but most were completely or partially top-killed and sprouted. For Arizona walnuts on sites that burned once, 30% survived as mature trees, 7% were killed, and 63% were partially to completely top-killed and sprouted. For Arizona walnuts on sites that burned once or twice, 30% survived as mature trees, 23% were killed, and 47% were partially to completely top-killed and sprouted [19]. See the Research Project Summary of this study for more information on the fire characteristics and response of Arizona walnut and associated trees (Arizona sycamore, desert-willow, Fremont cottonwood, and velvet ash) to these fires.
FUEL CHARACTERISTICS
Historically, fuels in low-elevation deserts of the Southwest were likely mostly sparse and discontinuous, although information on this is limited [77,125,251]. Keane et al. (2000) provide crown bulk density estimates for various structural stages of riparian forests and several other plant communities on the Gila National Forest. See the publication for further information [116].
Information on the flammability of Arizona walnut was lacking in the literature, but a few publications provide measures of live Arizona walnut fuels. Leaf area index is used in fuel models to help predict fire behavior and severity [231]. On the Balcones Canyonlands National Wildlife Refuge, Texas, stream bottom forests with Arizona walnut averaged 3.6 m²/m² in leaf area index, 329 g/m² in total area fuels, 112.2 g/m³ in canopy bulk density, and 297 g/m³ in maximum canopy bulk density [230].
Because the canopy and subcanopy layers are often well developed (see Plant Communities and Stand or Population Structure), fuels may be abundant in riparian woodlands with Arizona walnut [72]. The upper canopies of Emory oak/Arizona walnut communities of the Mogollon Rim are described as "luxurious and abundant", with well-developed shrub and herb layers and ladder fuels [13]. In a study across Arizona and New Mexico, ponderosa pine/Arizona walnut habitat types had 5% to >50% cover of canopy and subcanopy trees, >5% shrub cover (includes flammable junipers and oaks), and >25% herb cover (mostly bunchgrasses; e.g., squirreltail and western wheatgrass) [223]. Likewise, white fir/Arizona walnut communities of the Colorado Plateau and southern Arizona often have dense canopy and subcanopy layers, with well-developed shrub and herb layers [153].
Some communities with Arizona walnut have climbing ladder fuels [13]. Canyon grape [13,153,223], sweet mountain grape [177], and Virginia creeper [13]—which often grow into upper canopies—occur in communities in which Arizona walnut is dominant or important [13,153]. On the Fort Bowie National Historic Site, for example, canyon grape grows as a liana in Arizona walnut-netleaf hackberry-gum bully communities [247]. Reid et al. (2000) report that the combined cover of vines, lianas, and short shrubs in Arizona walnut-dominated communities of New Mexico ranges from 25% to 60% [181].
Nonnative species can alter fuel distributions and loads. When riparian vegetation composition and structure change as a result of nonnative species invasions into the overstory and/or understory, fuel characteristics may also change. In turn, this can alter fire intensity, severity, and/or rates of spread [251] (see Fire Regimes and Management Under a Changing Climate).
FIRE REGIMES
In shrub and mixed-deciduous riparian communities of the Southwest and northern Mexico, wildfires were historically ignited by lightning in June and July [15]. Contemporary fires often spread into riparian areas from adjacent uplands [19,56], but fires can also start in riparian zones [251]. It is uncertain how frequent fires were within riparian areas of these deserts historically. Fires in small drainages likely had different frequencies and behaviors than fires in larger drainages where riparian zones were wider and vegetation more developed [19,168]. Fire intervals may be long and severity low in riparian mixed-deciduous woodlands where periodic flooding scours the understory, limiting buildup of fine fuels and litter [251,252].
Information on historical fire regimes in riparian areas of the Southwest is limited due to lack of data [93,201,252], and the variability of historical fire regimes within and among desert riparian areas is not well known. The dynamic nature of riparian ecosystems makes their fire regimes hard to characterize [251,252]. Historically, sparse surface fuels and the variable patchiness of desert plant communities probably limited fire size, severity, and intensity, including fires in riparian zones [77,125,251]. Webb (2017, 2019) suggests that historical fires varied in severity and extent across southwestern riparian ecosystems. Most fires were likely patchy and of low severity due to the patchiness of vegetation in desert landscapes [127,251,252]; however, mixed- and high-severity fires likely occurred as well [127]. Fire might have been historically uncommon in some riparian areas [77,93,202,204,251,252]. In many cases, fires that started in the uplands might have slowed or stopped in riparian zones because temperatures were cooler and live fuel moistures higher [32,77,93,251]. For example, fires would often burn out when they reached borders of Arizona walnut-Arizona sycamore-velvet ash woodlands [62].
In many areas, stand structure and function of mixed-deciduous riparian woodlands have been significantly altered since European-American exploration and settlement began [251]. Before that time, American Indians likely burned riparian zones, although the extent of such burning is unknown [146,147]. Riparian areas experienced rapid ecological change [63,251,252] during European-American exploration (1540-1821) and settlement (1822-1912) periods [63]. In Arizona, for example, Spanish explorers imported livestock to the San Pedro Valley as early as the 1500s; European-Americans cut timber throughout the settlement period; miners diverted water from the San Pedro River to process ore in the 1800s; and fur trappers hunted American beavers until the animals were extirpated from the valley [251,252].
Changes in hydrology and vegetation have undoubtedly affected fire regimes of southwestern riparian communities and surrounding uplands. However, the degree to which fire regimes are changing—or what the effects might be for riparian ecosystem structure, function, and resilience—are not well known [251,252]. The greatest ecological change in riparian areas since large-scale livestock ranching was introduced is probably a shift in fire regimes from mostly patchy surface fires to larger crown fires [10,251]. Some research suggests that fire frequency and severity are increasing in some southwestern riparian ecosystems [41,42,75,80,93,108,220,222,252], although the extent of these increases are largely unknown. Wildfire became a significant disturbance agent in southwestern riparian areas the end of the 20th century. With the reduction in frequency and magnitude of floods, litter and debris accumulated in the forest understory. This accumulation, along with increased density of native and nonnative vegetation, resulted in greater fuel loads, fire sizes, and fire intensities than existed prior to riparian modification (review by [202]).
Factors that can increase fire severity include drought, altered flood disturbance, anthropogenic ignitions in high-use areas [32,41,75,80,222,251,258], flood and fire exclusion and associated high fuel loads, and more continuous fuels [32,75,80,93,96,251,258] resulting, in part, from spread of nonnative plant species [32,41,75,222,251]. Severe or intense fires can induce uniform changes in structure and composition across riparian zones by causing widespread mortality of native trees [14,251], increasing the likelihood of postfire establishment of nonnative plants [93,251,252]. Severe fires may be less common in areas with natural flood regimes [80,251,252].
Drought and/or human engineering can cause changes in hydrology (e.g., surface flows, groundwater levels, and channel and floodplain morphology) that favor nonnative plant species over native ones [42,75,93,108,183,252,261]. Nonnative annual grasses—particularly red brome, Mediterranean grasses, and cheatgrass [31,32]—and the nonnative trees Russian-olive [260] and tamarisk (saltcedar and five-stamen tamarisk [31,259] and potentially Athel tamarisk [242] and French tamarisk [43]) can increase fuel continuity and alter fire regimes in riparian communities of the Southwest [31,32] (see Fire Management Considerations). Fires may be larger, more frequent, and more severe in invaded communities [32]. For example, fire-induced mortality of native riparian trees can increase dramatically when tamarisk occurs in the prefire plant community [251]. On 30 sites across the Southwest, high fire consumption and postfire mortality of Fremont cottonwoods and willows were positively associated with high prefire tamarisk cover. Fire was more severe where tamarisk was present than where it was not, resulting in more tissue damage to native trees. When prefire tamarisk cover was >50%, fire completely consumed the fine tissues of all native trees present [75].
Presettlement fire regimes of mixed-deciduous riparian communities of the Southwest likely varied among sites [251,252]. Riparian pine-oak woodlands and forests in canyons might have facilitated fire spread between desert grasslands and higher-elevation forests [112,113]. LANDFIRE (2008) models classify fire regimes of Biophysical Settings dominated by cottonwood-willow communities in the Southwest into two groups: Fire Regime Group I (<35-year fire intervals, mixed severity) and Fire Regime Group V (>200-year fire intervals, replacement severity). Communities in the frequent-fire group are generally those along larger rivers, and those in the infrequent fire group are generally smaller desert riparian areas (i.e., stringers) [127].
Pine, oak, and mixed pine-oak communities in the Southwest likely experienced mostly frequent, low- and mixed-severity fires [113,127,184]. LANDFIRE (2008) models classify fire regimes of southwestern Madrean pine-oak-juniper and southern Rocky Mountain mixed-conifer Biophysical Settings in Fire Regime Groups I and III (35- to 200-year return intervals, low and mixed severity), although those are general models and not specific to riparian areas with Arizona walnut [127]. In the Rhyolite and Pine canyons of the Chiricahua Mountains, southeastern Arizona, presettlement point fire intervals (1700–1876) in mixed pine-oak woodlands (Apache pine, Arizona pine, Chihuahuan pine, Arizona oak, Emory oak, and silverleaf oak) ranged from 1 to 15 years and averaged 3 to 4 years [113].
See these FEIS publications for information on historical fire regimes in plant communities in which Arizona walnut is dominant or most common:
FIRE MANAGEMENT CONSIDERATIONS
There is limited research on the impacts of fire on southwestern riparian communities [252]. Dams, waterway diversions, and climate change have greatly altered the hydrology of many streams and rivers. In the absence of natural flooding and with lowering water tables, fire is becoming the dominant disturbance in some riparian ecosystems of the Southwest. Groundwater depth and streamflow greatly influence vegetation type and extent in riparian ecosystems [41,96,108,251], and fire regimes seem to be changing in some lowland riparian communities with altered hydrology. Changing fire regimes are likely to have drastic, potentially irreversible effects on biodiversity and function of affected riparian communities. Alterations in plant community structure and plant and animal community composition may occur, including declines in wildlife species of concern [252].
More severe, intense, and/or frequent fire can interact with altered hydrology to accelerate the replacement of native plants with nonnative plants [131,251]. In many riparian areas in the Southwest, there have been increases in tamarisk, Russian-olive, Mediterranean grass, and other nonnative plants that have different fuel characteristics, fire tolerances, and postfire fire responses than native species. Establishment and spread of tamarisk [185,259] and other nonnative species have changed fuel and fire regime characteristics in many riparian areas, and increased fire risk has been associated with high cover of tamarisk and Russian-olive [41,75,156,183,187,251,252]. Where groundwater levels have dropped, tamarisk has a competitive advantage over cottonwoods [131,251]. Its higher tolerance to drying soils [209] and frequent fire are driving changes in riparian stand structure and fire regimes, resulting in decreasing cover of formerly dominant, native trees such as Arizona walnut, cottonwoods, and willows [252,259]. Fire intervals in mixed-deciduous riparian woodlands may be as frequent as 15 years in areas where tamarisk cover is high [75,126,173,251,252]. Where natural flow regimes are more intact and tamarisk is suppressed by native vegetation, Arizona walnut and other native trees are more likely to dominate postfire landscapes [252].
Fire management in southwestern riparian areas may include fuel reduction and prescribed fire treatments that promote or maintain native vegetation [252] and reduce or eradicate invasives [198,251]. Although simulated fuel reduction treatments showed potential to mitigate fire severity in some mixed-deciduous woodlands and forests under projected climate change in the Huachuca Mountains, projected basal area and mortality rates in riparian zones were unchanged by simulated fuel treatments, and a reduction in basal area and spatial extent of riparian species occurred with or without fire and regardless of treatments [163]. Prescribed fire may be most effective when used in combination with other restoration, such as restoring natural waterway flows [252]. However, little information was available on using prescribed fire in riparian plant communities of the Southwest [15]. The effects of fire, including prescribed burning, have been better studied in southwestern grasslands and savannas than in adjacent riparian woodlands and forests [19,77,93,201], so more information is needed on the use of prescribed fire and postfire restoration in riparian areas. Fire may be severe, and lethal to overstory deciduous trees, in riparian woodlands that have not experienced fire for many decades.
Bock and Bock (1990) do not recommend prescribed fire in late-successional riparian woodlands of the Southwest because "fire is difficult to manage and potentially very destructive" in those habitats [18]. Refined fuel and fire behavior models are needed for riparian ecosystems of the Southwest, including models for areas in which tamarisk has become dominant. Webb et al. (2019) review the information available for using prescribed fire in southwestern riparian areas, with and without invasive species [252].
Burned areas with Arizona walnut may provide habitat for many species of wildlife. For example, peregrine falcons and other raptors may use new burns in Arizona sycamore-Arizona walnut woodlands as hunting grounds [84]. Along the Mimbres River in New Mexico, Arizona myotis used large, fire damaged Arizona walnut as day-roosting trees [234]. See Importance to Wildlife and Livestock for more information on this topic.FEDERAL LEGAL STATUS
None [238]
OTHER STATUS
Chinquapin oak-Arizona walnut-slippery elm/white crownbeard forests on the Edwards Plateau region of Texas are ranked as Imperiled or Vulnerable (G2 or G3) [158]. Other information on state- and province-level protection status of plants in the United States and Canada is available at
NatureServe.
IMPORTANCE TO WILDLIFE AND LIVESTOCK
A myriad of wildlife species use riparian habitats [64,65,101,137,148,246,249,252]. Riparian areas comprise a small part of total land area in the southwestern United States and northern Mexico, but they are extremely important to biotic communities [252]. Riparian areas are among the most diverse and productive ecosystems in desert bioregions, supporting floras and faunas that are biologically richer than adjacent uplands [34,47,54,252]. Riparian areas and washes provide water, cover, and breeding habitat for many species of wildlife, including obligate riparian species [92,148]. For wildlife that also use uplands, riparian areas provide refuge from the harsher, surrounding environments [148]. Wildlife that use riparian ecosystems for breeding, migration, and wintering habitat include large ungulate [64,252], small mammal, bird [6,64,101,182,221,249], reptile, amphibian, and arthropod species [35,40,64,182,252]. Riparian ecosystems provide habitat for many threatened or endangered species. Of 40 Federally listed Threatened or Endangered wildlife species in New Mexico—which includes several endemic species—at least 70% require aquatic and/or riparian habitat to feed, reproduce, and/or carry out their life cycles [235,252]. Riparian areas and washes also provide water, cover, and breeding habitat for livestock [38,92,148].
A variety of mammals use habitats with Arizona walnut [29]. Mixed-deciduous communities provide habitat for large and small mammals (e.g., plains bison [130] and rodents [71]). In the Northern Chihuahuan Desert, bison use a mosaic of habitats that include Arizona sycamore-Arizona walnut, oak-pine woodland, and plains grassland communities [130]. Mammals that use Arizona walnut-desert-willow communities on the Stockton Plateau include white-tailed deer, ringtails, eastern fox squirrels, and Merriam's pocket mice [103]. Streamside Arizona walnut communities provide habitat for Botta's pocket gophers [71] and Arizona gray squirrels [118].
Arizona walnut provides food for wildlife. Some bird [79,241] and many rodent species [30,79,241,246] consume the nuts [21], especially Arizona gray [58,218,219], Chiricahua fox [120], and eastern fox squirrels. Deer may browse the twigs and foliage [123,246], although Arizona walnut browse is not preferred. It provides minor summer and winter forage for desert mule deer [140]. Gleaning and probing birds forage on Arizona walnut. On the Sierra Ancha Experimental Forest, for example, the bridled titmouse and Hutton's vireo forage on Arizona walnut trees [182]. Arizona walnut catkins are generally not consumed by either vertebrates or invertebrates [111].
Arizona walnut provides breeding, wintering, and migratory corridors for a wide variety of bird species [23,47,101,225]. Riparian woodlands support disproportionately large bird populations, given their small area [225]. The structurally diverse, species-rich vegetation along many southwestern streams supports high densities of territories and nest sites for a variety of birds, including several species of high conservation priority [202]. The buff-breasted flycatcher [141], greater pewee [53], and the federally endangered [238] southwestern willow flycatcher use Fremont cottonwood-Arizona sycamore-Arizona walnut woodlands as nesting habitat [211]. The ferruginous pygmy-owl uses Arizona walnut-Arizona sycamore-velvet ash riparian woodlands habitats in southern and central Arizona, where the owl is at the northern end of its distribution [51]. Arizona sycamore-Arizona walnut woodlands are important sources of prey for peregrine falcons [84], and Cooper's hawks use and nest in Arizona white oak-Arizona sycamore-Arizona walnut communities. See these sources for a list of bird species that use riparian habitats with Arizona walnut as breeding habitat in Arizona: [23,47,101,221].
Herptiles that use riparian communities with Arizona walnut include the black-necked garter snake [137], Sonora mud turtle [100], lowland leopard frog [243], northern leopard frog, and canyon treefrog [137].
Walnut husk fly larvae feed nearly exclusively on the husks of Arizona walnut fruits and live part of their life cycle in litter and soil beneath Arizona walnut trees [61,164]. Arizona walnut is a preferred host for the walnut twig beetle, which broods in the bark of Arizona walnut branches [135].
Palatability and Nutritional Value
Arizona walnut tissues contain quinones [155] that make the browse unpalatable [107,190].
Walnut fruits are high in lipids and calories [39]. See these sources for information on the nutritional content of Arizona walnut browse: [48,49].
Cover Value
Arizona walnut provides hiding, resting, and/or nesting cover for many mammal and bird species [2,86]. Plant communities with Arizona walnut are often critical habitat for wildlife that use riparian areas for loafing cover and water but also use surrounding, harsher environments [86]. Squirrels nest in the forks of large Arizona walnut trees [86]. Arizona myotis roost in Arizona walnut during the day [234]. Acorn woodpeckers and other primary cavity nesters sometimes excavate the large branches and trunks of Arizona walnut for nesting cover [161]. However, Strong and Bock (1990) suggest that because Arizona walnut has very hard, decay-resistant wood, primary cavity nesters likely select other riparian tree species for excavation over Arizona walnut [221].
High-quality fish habitat depends on suitable water temperatures for spawning and survival [64,98]. Arizona walnut provides shade cover for fish, including the longfin dace [137] and the Federally Endangered [238] Rio Grande silvery minnow [57], and Apache and Gila trout [64].
VALUE FOR REHABILITATION OR RESTORATION
Arizona walnut is planted for restoration of riparian areas after flood damage and on sites that have lost native overstory trees [28]. It can be grown from seeds [21] but may be difficult to establish, especially on sites where the water table has lowered. Mature, deep-rooted Arizona walnut trees may persist on such sites, but seedlings may fail to establish [28]. A greenhouse study found Arizona walnut did not establish from cuttings; instead, it required tissue culture [169]. Bonner (2008) reviews propagation techniques for walnuts in general and Arizona walnut in particular [21].
OTHER USES
Riparian areas with Arizona walnut provide many ecosystem services including recreational opportunities, harboring pollinators, providing livestock forage [200,252], and enhancing water quality [252]. Riparian areas reduce sedimentation of stream channels, maintain streambanks stability, and filter excess nutrients that occur in runoff water [32,205]. Riparian vegetation reduces nonpoint source pollution in waterways by filtering sediments, chemicals, and nutrients from overflow. The roots of streamside plants help prevent excessive erosion during floods [236] and promote groundwater infiltration [85], and vegetation surrounding active channels helps slow water velocity during floods [236]. Riparian trees and shrubs shade surface water, reducing water temperatures [236] and loss to evaporation [252].
Arizona walnut is not an important timber tree due to its limited distribution and small bole size [79]. The wood is hard [221] and durable and is used for posts, furniture, gunstocks, and veneer [16,81,132,171,241]. It has high oil content [48,49] and makes good firewood, but it is not a commercial species [1]. Paradox walnut, an Arizona walnut hybrid, is used as rootstock for commercial English walnuts [206]. See Miller (1976) for characteristics of Arizona walnut wood [149].
Arizona walnut is planted as a shade tree [79,117,132,254]. The nut husks are used to make dye [81,160,188]. Arizona walnut produces napthalene [155], a natural insecticide [162], and benzohydroquinone [155], which is used as a skin lightener [22].
The nuts are edible [21,52,81,121,132] and eaten by all peoples. They are a traditional food of indigenous peoples of the Southwest [52,81,188].
ADDITIONAL MANAGMENT CONSIDERATIONS
The value of southwestern riparian woodlands for both biotic communities and human livelihoods is well established [64,65,104,157,202,214,240], and maintaining and/or restoring riparian areas of the Southwest is a critical management issue [64,65,143,202]. Management goals for southwestern riparian areas may include restoring floodplains, safeguarding high-quality riparian areas, reducing hazardous fuels, preventing spread of fire, and preventing establishment and spread of nonnative invasive plants [198,251].
Many riparian communities of the Southwest are highly imperiled due to anthropogenic disturbances [200]. Few naturally functioning riparian areas remain in the region, and those that do are threatened with climate change [148,252]. Within the last 100 to 150 years, humans have modified many riparian areas extensively by irrigating, pumping groundwater, building dams and diversions, clearing floodplains for agriculture [27,97] and development [110], cutting wood, using off-highway vehicles [90], grazing livestock [36,144], and spreading nonnative invasive plants. These modifications have resulted in significant changes in the physical environment and biota [82,148,150,215,252]. For example, livestock generally use riparian areas more than adjacent, less productive upland communities, compacting soils and reducing recruitment of palatable deciduous seedlings and saplings into the overstory [60,148]. Use of the unpalatable Arizona walnut by livestock generally indicates overbrowsing or overstocking [239,248].
Altered hydrological regimes are often associated with establishment and spread of nonnative plants. Altered hydrology and sediment deposition resulting from river impoundment may reduce or eliminate germination and recruitment of native deciduous trees while promoting germination and recruitment of tamarisk [252]. Consequently, tamarisk species have established in many riparian areas of the Southwest [9,62,148,183,185,216,220,260] (see Fire Management Considerations). Many riparian areas have also been invaded by nonnative Russian-olive. In Canyon de Chelly National Monument, Arizona, cover of native trees, including Arizona walnut, increased within 2 years of removal of Russian-olive and tamarisk (saltcedar, five-stamen tamarisk, and their hybrids) [183]. Maintaining an ecologically healthy riparian community can help stop establishment and spread of tamarisk and other invasive plant species [7]. Dense shade in closed-canopy communities with Arizona walnut may inhibit establishment of tamarisk [68].
Flow regimes that fall within the natural range of variability for undammed waterways can have multiple benefits, including:Arizona walnut generally persists with grazing [11,144]; in part, due to its unpalatability. However, near Little Ash Creek on the Prescott National Forest, Arizona, Arizona walnut was present only in livestock exclosures [229], suggesting that trampling and/or some browsing of Arizona walnut had occurred outside the exclosures.
Arizona walnut may spread infectious fungi to cultivated English walnuts. Walnut anthrocnose, a leaf blight, may spread from Arizona walnut to English walnuts. A common garden study found that among eight native or cultivated (nonnative) walnut species, Arizona walnut was most susceptible to walnut anthrocnose infection [17]. In the laboratory, Arizona walnut seedlings were "highly susceptible" to infection by black hop root rot, a root-rot fungus [142]. The walnut twig beetle vectors infection of thousand cankers disease, which also infects English walnuts [135].
MANAGEMENT UNDER A CHANGING CLIMATE
Southwestern riparian ecosystems are vulnerable to decline under hotter and drier conditions [25,46,62,214,251,252]. Riparian woodlands with Arizona walnut have been rapidly dwindling as water tables have been rapidly lowering [136]. Climate change and variation—and associated impacts on hydrology, channel morphology, and riparian vegetation—may alter fire regimes, increasing the likelihood of more frequent or severe wildfires [19,77,203,252]. Prolonged drought may increase fire risk by decreasing water availability and reducing fuel moisture [168,251,252].
Changes in water flow are expected with climate change, including lower water tables and increased frequency and severity of extreme weather that will likely result in more frequent and severe floods as well as more intense droughts [78,93,193,201,202,251]. Warming in montane areas may increase the number of severe floods downstream by increasing the rate of snowmelt into downstream waterways such as the Middle Rio Grande [251]. Monsoons may occur later in the year and with greater severity [202]. Droughts are likely to be increasingly severe and prolonged, resulting in further reductions in discharge volume [202].
Distribution and composition of upland communities may change with climate warming [92,251,252]. In turn, this may affect composition of riparian vegetation in ways that increase fire risk (e.g., facilitating spread of nonnative species [201]). Climate change could alter competitive relationships between Arizona walnut and other native species, and also between Arizona walnut and nonnative species such as tamarisk and Russian-olive [156].| Table A1—Common and scientific names of fungi and plants mentioned in this review. Links go to FEIS Species Reviews. | |
| Common name | Scientific name |
| Fungi | |
| black hop root rota | Phytophthora citricola |
| thousand cankers | Geosmithia morbida |
| walnut anthrocnosea | Ophiognomonia leptostyla |
| Forbs | |
| white crownbeard | Verbesina virginica |
| Graminoids | |
| cheatgrassa | Bromus tectorum |
| Mediterranean grassa | Schismus spp. |
| red bromea | Bromus rubens |
| sideoats grama | Bouteloua curtipendula |
| squirreltail | Elymus elymoides |
| western wheatgrass | Pascopyrum smithii |
| Shrubs | |
| buckthorn | Rhamnus spp. |
| California brickellbrush | Brickellia californica |
| catclaw acacia | Senegalia greggii |
| false indigo bush | Amorpha fruticosa |
| gum bully | Sideroxylon lanuginosa |
| mesquite | Prosopis spp. |
| mule-fat | Baccharis salicifolia |
| skunkbush sumac | Rhus trilobata |
| Trees | |
| alligator juniper | Juniperus deppeana |
| Apache pine | Pinus engelmannii |
| Arizona cypress | Hesperocyparis arizonica |
| Arizona alder | Alnus oblongifolia |
| Arizona pine | Pinus arizonica |
| Arizona sycamore | Platanus wrightii |
| Arizona walnut | Juglans major |
| Arizona white oak | Quercus arizonica |
| Ashe's juniper | Juniperus ashei |
| bald cypress | Taxodium distichum |
| black cherry | Prunus serotina |
| blackjack oak | Quercus marilandica |
| black walnut | Juglans nigra |
| box elder | Acer negundo |
| Buckley's oak | Quercus buckleyi |
| canyon maple | Acer grandidentatum var. sinuosum |
| cedar-elm | Ulmus crassifolia |
| Chihuahuan pine | Pinus leiophylla var. chihuahuana |
| chinquapin oak | Quercus muehlenbergii |
| cottonwood | Populus spp. |
| cypress | Cupressaceae, includes Hesperocyparis and Taxodium spp. |
| desert-willow | Chilopsis linearis |
| eastern redcedar | Juniperus virginiana |
| Emory oak | Quercus emoryi |
| English walnuta | Juglans regia |
| five-stamen tamariska | Tamarix chinensis |
| Fremont cottonwood | Populus fremontii |
| Gambel oak | Quercus gambelii |
| Goodding's willow | Salix gooddingii |
| green ash | Fraxinus pennsylvanica |
| juniper | Juniperus spp. |
| lanceleaf cottonwood | Populus acuminata |
| little walnut | Juglans microcarpa |
| narrowleaf cottonwood | Populus angusitifolia |
| narrowleaf willow | Salix exigua |
| netleaf hackberry | Celtis laevigata var. reticulata |
| northern California walnut | Juglans hindsii |
| oak | Quercus spp. |
| Paradox hybrid walnut | Juglans hindsii × Juglans regia |
| pecan | Carya illinoensis |
| pine | Pinus spp. |
| pinyon | Pinus, subsection Cembroides |
| post oak | Quercus stellata |
| ponderosa pine (southwestern, Rocky Mountain) |
Pinus ponderosa var. brachyptera, Pinus ponderosa var. scopularum |
| Russian-olivea | Elaeagnus angustifolia |
| saltcedara | Tamarix ramosissima |
| silverleaf oak | Quercus hypoleucoides |
| slippery elm | Ulmus rubra |
| tamariska | Tamarix spp. |
| Texas live oak | Quercus fusiformis |
| twoneedle pinyon | Pinus edulis |
| velvet ash | Fraxinus velutina |
| walnut | Juglans spp. |
| western soapberry | Sapindus saponaria var. drummondii |
| white fir | Abies concolor var. glauca |
| willow | Salix spp. |
| Liana | |
| canyon grape | Vitis arizonica |
| eastern poison-ivy | Toxicodendron radicans |
| sweet mountain grape | Vitis monticola |
| Virginia creeper | Parthenocissus quinquefolia |
| aNonnative species. | |
| Table A2—Common and scientific names of wildlife species mentioned in this review. Links go to FEIS Species Reviews. | |
| Common name | Scientific name |
| Insects | |
| walnut husk fly | Rhagoletis juglandis |
| walnut twig beetle | Pityophthorus juglandis |
| Fish | |
| Apache trout | Oncorhynchus apache |
| Gila trout | Oncorhynchus gilae |
| longfin dace | Agosia chrysogaster |
| Rio Grande silvery minnow | Hybognathus amarus |
| Amphibians | |
| canyon treefrog | Hyla arenicolor |
| northern leopard frog | Lithobates pipiens |
| lowland leopard frog | Lithobates yavapaiensis |
| Reptiles | |
| black-necked garter snake | Thamnophis cyrtopsis |
| Sonora mud turtle | Kinosternon sonoriense |
| Birds | |
| acorn woodpecker | Melanerpes formicivorus |
| bridled titmouse | Baeolophus wollweberi |
| buff-breasted flycatcher | Empidonax fulvifrons |
| Cooper's hawk | Accipiter cooperii |
| ferruginous pygmy-owl | Glaucidium brasilianum |
| greater pewee | Contopus pertinax |
| Hutton's vireo | Vireo huttoni |
| peregrine falcon | Falco peregrinus |
| southwestern willow flycatcher | Empidonax traillii extimus |
| Mammals | |
| American beaver | Castor canadensis |
| Arizona gray squirrel | Sciurus arizonensis |
| Arizona myotis | Myotis occultus |
| Botta's pocket gopher | Thomomys bottae |
| Chiricahua fox squirrel | Sciurus nayaritensis chiricahuae |
| desert mule deer | Odocoileus hemionus crooki |
| eastern fox squirrel | Sciurus niger |
| Merriam's pocket mouse | Perognathus merriami |
| ringtail | Bassariscus astutus |
| squirrels | Sciuridae |
| white-tailed deer | Odocoileus virginianus |
| Table A3—Representative plant community classifications in which Arizona walnut may occur. | |
| FRES Ecosystems | |
| FRES14 Oak-pine | |
| FRES 16 Oak-gum-cypress | |
| FRES21 Ponderosa pine | |
| FRES 32 Texas savanna | |
| FRES33 Southwestern shrubsteppe | |
| FRES 34 Chaparral-mountain shrub | |
| FRES35 Pinyon-juniper | |
| FRES38 Plains grasslands [95] | |
| Kuchler Plant Associations | |
| K019 Arizona pine forest | |
| K023 Juniper-pinyon woodland | |
| K031 Oak-juniper woodland | |
| K058 Grama-tobosa shrubsteppe | |
| K066 Wheatgrass-needlegrass [122] | |
| SAF Cover Types | |
| 40 Post oak-blackjack oak | |
| 46 Eastern redcedar | |
| 66 Ashe juniper-redberry (Pinchot) juniper | |
| 101 Baldcypress | |
| 211 White fir | |
| 235 Cottonwood-willow | |
| 237 Interior ponderosa pine | |
| 239 Pinyon-juniper | |
| 240 Arizona cypress | |
| 241 Western live oak [83] | |
| SRM (Rangeland) Cover Types | |
| 413 Gambel oak | |
| 504 Juniper-pinyon pine | |
| 505 Grama-tobosa shrub | |
| 607 Wheatgrass-needlegrass | |
| 610 Wheatgrass | |
| 733 Juniper-oak | |
| 735 Sideoats grama-sumac-juniper [196] | |
1. Alden, Harry A. 1995. Hardwoods of North America. Gen. Tech. Rep. FPL-GTR-83. Madison, WI: U.S. Department of Agriculture, Forest Service, Forest Products Laboratory. 136 p. Available online: http://www.fpl.fs.usda.gov/documnts/fplgtr/fplgtr83.pdf [2004, January 6]. [46270]
2. Alexander, Billy G., Jr.; Ronco, Frank, Jr.; Fitzhugh, E. Lee; Ludwig, John A. 1984. A classification of forest habitat types of the Lincoln National Forest, New Mexico. Gen. Tech. Rep. RM-104. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 29 p. [300]
3. Alexander, Robert R. 1988. Forest vegetation on national forests in the Rocky Mountain and Intermountain regions: Habitat and community types. Gen. Tech. Rep. RM-162. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 47 p. [5903]
4. Alexander, Robert R.; Ronco, Frank, Jr. 1987. Classification of the forest vegetation on the National Forests of Arizona and New Mexico. Res. Note RM-469. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 10 p. [3515]
5. Allred, Kelly W.; Jercinovic, Eugene M.; DeWitt, Ivey. 2020. Flora Neomexicana III: An illustrated identification Manual; Part 2: Dicotyledonous plants. 2nd ed. Independently published. 795 p. [94735]
6. Anderson, Bertin W.; Ohmart, Robert D. 1977. Vegetation structure and bird use in the Lower Colorado River Valley. In: Johnson, R. Roy; Jones, Dale A., tech. coords. Importance, preservation and management of riparian habitat: A symposium; 1997 July 9; Tucson, AZ. Gen. Tech. Rep. RM-43. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 23-34. [52882]
7. Anderson, Bertin. 1998. The case for salt cedar. Restoration and Management Notes. 16(2): 130-134. [44007]
8. Aradhya, Mallikarjuna K.; Potter, Daniel; Simon, Charles J. 2006. Cladistic biogeography of Juglans (Juglandaceae) based on chloroplast DNA intergenic spacer sequences. In: Motley, Timothy J; Zerega, Nyree; Cross, Hugh, eds. Darwin's harvest: New approaches to the origins, evolution, and conservation of crops. New York: Columbia University Press: 143-170. [96265]
9. Bagstad, K. J.; Lite, S. J.; Stromberg, J. C. 2006. Vegetation, soils, and hydrogeomorphology of riparian patch types of a dryland river. Western North American Naturalist. 66(1): 23-44. [62270]
10. Bahre, Conrad J. 1995. Human disturbance and vegetation in Arizona's Chiricahua Mountains in 1902. Desert Plants. 11(4): 41-45. [26028]
11. Barstad, Janet Fisher. 1981. Factors controlling plant distribution in southeastern Arizona. Tempe, AZ: Arizona State University. 176 p. Thesis. [88293]
12. Baskin, Carol C.; Baskin, Jerry M. 2001. Seeds: Ecology, biogeography, and evolution of dormancy and germination. San Diego, CA: Academic Press. 666 p. [60775]
13. Bassett, Dick; Larson, Milo; Moir, Will. 1987. Forest and woodland habitat types (plant associations) of Arizona south of the Mogollon Rim and southwestern New Mexico. 2nd ed. Albuquerque, NM: U.S. Department of Agriculture, Forest Service, Southwestern Region. Variously paginated. [20308]
14. Bendix, Jacob; Cowell, C. Mark. 2010. Impacts of wildfire on the composition and structure of riparian forests in southern California. Ecosystems. 13(1): 99-107. [81845]
15. Bennett, Peter S.; Kunzmann, Michael R. 1992. The applicability of generalized fire prescriptions to burning of Madrean evergreen forest and woodland. Journal of the Arizona-Nevada Academy of Science. 24-25: 79-84. [18324]
16. Benson, Lyman; Darrow, Robert A. 1981. The trees and shrubs of the Southwestern deserts. Tucson, AZ: The University of Arizona Press. 416 p. [18066]
17. Black, W. M.; Neely, Dan. 1978. Relative resistance of Juglans species and hybrids to walnut anthracnose. Plant Disease Reporter. 62(6): 497-499. [11559]
18. Bock, Carl E.; Bock, Jane H. 1990. Effects of fire on wildlife in southwestern lowland habitats. In: Krammes, J. S., technical coordinator. Effects of fire management of southwestern natural resources: Proceedings of the symposium; 1988 November 15-17; Tucson, AZ. Gen. Tech. Rep. RM-191. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 50-64. [11273]
19. Bock, Carl E.; Bock, Jane H. 2014. Effects of wildfire on riparian trees in southeastern Arizona. The Southwestern Naturalist. 59(4): 568-574. [89514]
20. Boles, Patrick H.; Dick-Peddie, William A. 1983. Woody riparian vegetation patterns on a segment of the Mimbres River in southwestern New Mexico. The Southwestern Naturalist. 28(1): 81-87. [65317]
21. Bonner, Franklin T. 2008. Juglans L.: walnut. In: Bonner, Franklin T.; Karrfalt, Robert P., eds. Woody plant seed manual. Agric. Handbook No. 727. Washington, DC: U.S. Department of Agriculture, Forest Service: 601-606. [79265]
22. Boyle, J.; Kennedy, C. T. C. 1986. Hydroquinone concentrations in skin lightening creams. British Journal of Dermatology. 114(4): 501-504. [96059]
23. Brand, Ariana; Stromberg, Juliet C.; Noon, Barry R. 2010. Avian density and nest survival on the San Pedro River: Importance of vegetation type and hydrologic regime. Journal of Wildlife Management. 74(4): 739-754. [96266]
24. Brian, Nancy J. 1992. Historical review of water flow and riparian vegetation at Walnut Canyon National Monument, Arizona. Tech. Rep. NPS/WRUA/NRTR-92/44. Tucson, AZ: The University of Arizona, School of Renewable Natural Resources, Cooperative National Park Resources Studies Unit. 39 p. [19870]
25. Briggs, Mark K. 1996. Considering the damaged riparian area from a watershed perspective. In: Briggs, Mark K. Riparian ecosystem recovery in arid lands: Strategies and references. Tucson, AZ: The University of Arizona Press: 13-25. [44783]
26. Briggs, Mark K. 1996. Natural recovery in riparian ecosystems. In: Briggs, Mark K. Riparian ecosystem recovery in arid lands: Strategies and references. Tucson, AZ: The University of Arizona Press: 45-60. [44785]
27. Briggs, Mark K. 1996. Riparian ecosystem recovery in arid lands: Strategies and references. Tucson, AZ: The University of Arizona Press. 159 p. [44789]
28. Briggs, Mark K. 1996. Water availability. In: Briggs, Mark K. Riparian ecosystem recovery in arid lands: Strategies and references. Tucson, AZ: The University of Arizona Press: 61-77. [44786]
29. Brinkman, Kenneth A. 1974. Juglans L. walnut. In: Schopmeyer, C. S., ed. Seeds of woody plants in the United States. Agric. Handb. 450. Washington, DC: U.S. Department of Agriculture, Forest Service: 454-459. [7684]
30. Brock, John H. 1994. Phenology and stand composition of woody riparian plants in the southwestern United States. Desert Plants. 11(1): 23-31. [24155]
31. Brooks, Matthew L. 2008. Plant invasions and fire regimes. In: Zouhar, Kristin; Smith, Jane Kapler; Sutherland, Steve; Brooks, Matthew L., eds. Wildland fire in ecosystems: Fire and nonnative invasive plants. Gen. Tech. Rep. RMRS-GTR-42, Vol. 6. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 33-45. [70467]
32. Brooks, Matthew L.; Minnich, Richard A.; Matchett, John R. 2018. Southeastern deserts bioregion. In: van Wagtendonk, Jan W.; Sugihara, Neil G.; Stephens, Scott L.; Thode, Andrea E.; Shaffer, Kevin E.; Fites-Kaufman, Jo Ann, eds. Fire in California's ecosystems. 2nd ed. Oakland, CA: University of California Press: 353-378. [93914]
33. Brown, David E., ed. 1982. Biotic communities of the American Southwest--United States and Mexico. Desert Plants: Special Issue. Tucson, AZ: University of Arizona Press. 4(1-4): 1-342. [62041]
34. Brown, David E.; Lowe, Charles H.; Hausler, Janet F. 1977. Southwestern riparian communities: Their biotic importance and management in Arizona. In: Johnson, R. Roy; Jones, Dale A., tech. coords. Importance, preservation and management of riparian habitat: A symposium: Proceedings; 1977 July 9; Tucson, AZ. Gen. Tech. Rep. RM-43. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 201-211. [5348]
35. Brown, James H.; Kodric-Brown, Astrid; Whitham, Thomas G.; Bond, Hedley W. 1981. Competition between hummingbirds and insects for the nectar of two species of shrubs. The Southwestern Naturalist. 26(2): 133-145. [12236]
36. Brunelle, A.; Minckley, T. A.; Delgadillo, J.; Blissett, S. 2014. A long-term perspective on woody plant encroachment in the desert southwest, New Mexico, USA. Journal of Vegetation Science. 25(3): 829-838. [96267]
37. Buechner, Helmut Karl. 1944. The range vegetation of Kerr County, Texas, in relation to livestock and white-tailed deer. The American Midland Naturalist. 31(3): 697-743. [62475]
38. Buegge, J. Jeremy. 2001. Flora of the Santa Teresa Mountains in Graham County, Arizona. Journal of the Arizona-Nevada Academy of Science. 33(2): 132-149. [45078]
39. Burns, Thomas A.; Viers, Charles E., Jr. 1973. Caloric and moisture content values of selected fruits and mast. The Journal of Wildlife Management. 37(4): 585-587. [41689]
40. Bury, R. Bruce; Germano, David J.; Van Devender, Thomas R.; Martin, Brent E. 2002. The desert tortoise in Mexico. In: Van Devender, Thomas R., ed. The Sonoran desert tortoise: Natural history, biology, and conservation. Arizona-Sonora Desert Museum Studies in Natural History. Tucson, AZ: The University of Arizona Press; The Arizona-Sonora Desert Museum: 86-108. [69904]
41. Busch, David E. 1995. Effects of fire on southwestern riparian plant community structure. The Southwestern Naturalist. 40(3): 259-267. [26498]
42. Busch, David E.; Smith, Stanley D. 1993. Effects of fire on water and salinity relations of riparian woody taxa. Oecologia. 94(2): 186-194. [88320]
43. California Invasive Pest Council. 2022. Cal-IPC: Plants A to Z. In: Cal-IPC, [Online]. Berkeley, CA: California Invasive Pest Council (Producer). Available: https://www.cal-ipc.org/plants/profiles/ [2020, November 22]. [94821]
44. Campbell, C. J. 1973. Pressure bomb measurements indicate water availability in a southwestern riparian community. Res. Note RM-246. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 4 p. [11155]
45. Campbell, C. J.; Green, Win. 1968. Perpetual succession of stream-channel vegetation in a semiarid region. Journal of the Arizona Academy of Science. 5(2): 86-90. [53565]
46. Capon, Samantha J.; Chambers, Lynda E.; Mac Nally, Ralph; Naiman, Robert J.; Davies, Peter; Marshall, Nadine; Pittock, Jamie; Reid, Michael; Capon, Timothy; Douglas, Michael; Catford, Jane; Baldwin, Darren S.; Stewardson, Michael; Roberts, Jane; Parsons, Meg; Williams, Stephen E. 2013. Riparian ecosystems in the 21st century: Hotspots for climate change adaptation? Ecosystems. 16(3): 359-381. [96207]
47. Carothers, Steven W.; Johnson, R. Roy; Aitchison, Stewart W. 1974. Population structure and social organization of southwestern riparian birds. American Zoologist. 14(1): 97-108. [24192]
48. Carr, M. E.; Phillips, B. S.; Bagby, M. O. 1985. Xerophytic species evaluated for renewable energy resources. Economic Botany. 39(4): 505-513. [71932]
49. Carr, Merle E.; Mason, Charles T., Jr.; Bagby, Marvin O. 1986. Renewable resources from Arizona trees and shrubs. Forest Ecology and Management. 16(1-4): 155-167. [3053]
50. Carter, Jack L. 1997. Trees and shrubs of New Mexico. Boulder, CO: Johnson Books. 534 p. [72647]
51. Cartron, Jean-Luc E.; Stoleson, Scott H.; Russell, Stephen M.; Proudfoot, Glenn A.; Richardson, W. Scott. 2000. The ferruginous pygmy-owl in the tropics and at the northern end of its range: Habitat relations and requirements. In: Cartron, Jean-Luc E.; Finch, Deborah M., tech. eds. Ecology and conservation of the cactus ferruginous pygmy-owl in Arizona. Gen. Tech. Rep. RMRS-GTR-43. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 47-55. [35492]
52. Castetter, Edward F. 1935. Ethnobiological studies in the American Southwest. I. Uncultivated native plants used as sources of food. Biological Series No. 4: Volume 1. Albuquerque, NM: University of New Mexico. 62 p. [35938]
53. Chace, Jameson F.; McKinney, Shawn T.; Cruz, Alexander. 2000. Nest-site characteristics and nesting success of the greater pewee in Arizona. The Southwestern Naturalist. 45(2): 169-275. [35930]
54. Chaney, Ed; Elmore, Wayne; Platts, William S. 1990. Livestock grazing on western riparian areas. Eagle, ID: Northwest Resource Information Center, Inc. Produced for the U.S. Environmental Protection Agency. 45 p. [17790]
55. Clover, Elzada U.; Jotter, Lois. 1944. Floristic studies in the Canyon of the Colorado and tributaries. The American Midland Naturalist. 32(3): 591-642. [62472]
56. Coffman, Gretchen C.; Ambrose, Richard F.; Rundel, Philip W. 2010. Wildfire promotes dominance of invasive giant reed (Arundo donax) in riparian ecosystems. Biological Invasions. 12(8): 2723-2734. [82396]
57. Coleman, Ross; Hutson, Alison M.; Toya, Louie A.; Tave, Douglas. 2011. Using native plants to provide natural ecosystem functions in a conservation fish hatchery. Native Plants Journal. 12(3): 216-225. [95950]
58. Cudworth, Nichole L.; Koprowski, John L. 2013. Foraging and reproductive behavior of Arizona gray squirrels (Sciurus arizonensis): Impacts of climatic variation. Journal of Mammalogy. 94(3): 683-690. [96237]
59. Danzer, Steven J.; Jemison, Roy; Guertin, D. Phillip. 2001. Riparian plant communities in the mountains of southeastern Arizona. The Southwestern Naturalist. 46(2): 191-199. [40147]
60. Davis, Gary A. 1977. Management alternatives for the riparian habitat in the Southwest. In: Johnson, Roy; Jones, Dale A., technical coordinators. Importance, preservation and management of riparian habitat: A symposium; 1977 July 9; Tucson, AZ. General Technical Report RM-43. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 59-67. Available from: NTIS, Springfield, VA 22151; PB-274 582. [5336]
61. Davis, Jeremy M.; Coogan, Laura E.; Papaj, Daniel R. 2015. Big maggots dig deeper: Size-dependent larval dispersal in flies. Oecologia. 179(1): 55-62. [96271]
62. DeBano, Leonard F.; Baker, Malchus B., Jr. 1999. Riparian ecosystems in southwestern United States. In: Ffolliott, Peter F.; Ortega-Rubio, Alfredo, eds. Ecology and management of forests, woodlands, and shrublands in the dryland regions of the United States and Mexico: Perspectives for the 21st century. Co-edition No. 1. Tucson, AZ: The University of Arizona; La Paz, Mexico: Centro de Investigaciones Biologicas del Noroeste, SC; Flagstaff, AZ: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 107-120. [37054]
63. DeBano, Leonard F.; Ffolliott, Peter F. 2002. Riparian history of the Southwest. In: Hydrology and water resources in Arizona and the Southwest: Proceedings, Arizona-Nevada Academy of Science--Hydrology section; 2002 April 6; Glendale, AZ. Volume 32. Mesa, AZ: Arizona-Nevada Academy of Science: 33-38. [45005]
64. DeBano, Leonard F.; Rinne, John N.; Baker, Malchus B., Jr. 2003. Management of natural resources in riparian corridors. Journal of the Arizona-Nevada Academy of Science. 35(1): 58-70. [96273]
65. DeBano, Leonard F.; Schmidt, Larry J. 1989. Improving southwestern riparian areas through watershed management. Gen. Tech. Rep. RM-182. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 33 p. [11016]
66. DeBano, Leonard F.; Schmidt, Larry J. 2004. Definitions and classifications. Chapter 2. In: Baker, Jr., Malchus B.; Ffolliott, Peter F.; DeBano, Leonard F.; Neary, Daniel G., eds. Riparian areas of the southwestern United States: Hydrology, ecology, and management. Washington, DC: Lewis Publishers: 11-27. [89631]
67. DeVelice, Robert L.; Ludwig, John A. 1983. Forest habitat types south of the Mogollon Rim, Arizona and New Mexico. Final report: Cooperative Agreement No. 28-K2-240. Las Cruces, NM: New Mexico State University. 47 p. [780]
68. Dewine, J. M.; Cooper, D. J. 2008. Canopy shade and the successional replacement of tamarisk by native box elder. Journal of Applied Ecology. 45(2): 505-514. [70231]
69. Diamond, David D. 1997. An old-growth definition for western juniper woodlands: Texas ashe juniper dominated or codominated communities. Gen. Tech. Rep. SRS-15. Asheville, NC: U.S. Department of Agriculture, Forest Service, Southern Research Station. 10 p. [28040]
70. Diamond, David D.; Rowell, Gareth A.; Keddy-Hector, Dean P. 1995. Conservation of ashe juniper (Juniperus ashei Buchholz) woodlands of the central Texas Hill Country. Natural Areas Journal. 15(2): 189-197. [26519]
71. Dice, Lee R. 1942. Ecological distribution of Peromyscus and Neotoma in parts of southern New Mexico. Ecology. 23(2): 199-208. [70346]
72. Dick-Peddie, William A. 1993. New Mexico vegetation: Past, present, and future. Albuquerque, NM: University of New Mexico Press. 244 p. [21097]
73. Diggs, George M., Jr.; Lipscomb, Barney L.; O' Kennon, Robert J. 1999. Illustrated flora of north-central Texas. Sida Botanical Miscellany, No. 16. Fort Worth, TX: Botanical Research Institute of Texas. 1626 p. [35698]
74. Donart, Gary B.; Sylvester, Donell; Hickey, Wayne. 1978. A vegetation classification system for New Mexico, U.S.A. In: Hyder, Donald N., ed. Proceedings, 1st international rangeland congress; 1978 August 14-18; Denver, CO. Denver, CO: Society for Range Management: 488-490. [4094]
75. Drus, Gail Michelle. 2013. Tamarisk (Tamarix spp.) and desert riparian ecosystem change. Santa Barbara, CA: University of California, Santa Barbara. 173 p. Dissertation. [88329]
76. Durkin, Paula; Muldavin, Esteban; Bradley, Mike; Carr, Stacey E. 1996. A preliminary riparian/wetland vegetation community classification of the upper and middle Rio Grande watersheds in New Mexico. In: Shaw, Douglas W.; Finch, Deborah M., technical coordinators. Desired future conditions for southwestern riparian ecosystems: Bringing interests and concerns together: Proceedings; 1995 September 18-22; Albuquerque, NM. Gen. Tech. Rep. RM-GTR-272. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 44-57. [26192]
77. Dwire, Kathleen A.; Kauffman, J. Boone. 2003. Fire and riparian ecosystems in landscapes of the western USA. In: Young, Michael K.; Gresswell, Robert E.; Luce, Charles H., eds. Selected papers from an international symposium on effects of wildland fire on aquatic ecosystems in the western USA; 2002 April 22-24; Boise, ID. In: Forest Ecology and Management. 178(1-2): 61-74. [44923]
78. Easterling, David R.; Meehl, Gerald A.; Parmesan, Camille; Changnon, Stanley A.; Karl, Thomas R.; Mearns, Linda O. 2000. Climate extremes: Observations, modeling, and impacts. Science. 289(5487): 2068-2047. [96228]
79. Elias, Thomas S. 1980. The complete trees of North America: Field guide and natural history. New York: Times Mirror Magazines, Inc. 948 p. [21987]
80. Ellis, Lisa M. 2001. Short-term response of woody plants to fire in a Rio Grande riparian forest, central New Mexico, USA. Biological Conservation. 97(2): 159-170. [38945]
81. Elmore, Francis H. 1944. Ethnobotany of the Navajo. Monograph Series: 1(7). Albuquerque, NM: University of New Mexico. 136 p. [35897]
82. Espinoza, Jose Javier Ochoa; Ayala, Cesar Cantu; Castillon, Eduardo Estrada; Saldivar, Fernando Gonzalez; Sauceda, Jose Uvalle; Jurado, Enrique; ChapaVargas, Leonardo; Jaramillo, Edmar Melendez; Hernandez, Edgardo Ortiz. 2017. Livestock effect on floristic composition and vegetation structure of two desert scrublands In Northwest Coahuila, Mexico. The Southwestern Naturalist. 62(2): 138-145. [95955]
83. Eyre, F. H., ed. 1980. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters. 148 p. [905]
84. Falxa, Gary A. 1976. Peregrine falcon nesting survey and habitat evaluation in the Gila National Forest. USDA Forest Service Wildlife Technical Bulletin 3; Chihuahuan Desert Research Institute Contribution 3. Alpine, TX: Chihuahuan Desert Research Institute. 31 p. [71179]
85. Ffolliott, P. F.; Baker, M. B., Jr.; DeBano, L. F.; Neary, D. G. 2004. Introduction. In: Baker, Jr., Malchus B.; Ffolliott, Peter F.; DeBano, Leonard F.; Neary, Daniel G., eds. Riparian areas of the southwestern United States: Hydrology, ecology, and management. Washington, DC: Lewis Publishers: 1-8. [96192]
86. Ffolliott, Peter F. 2002. Ecology and management of evergreen oak woodlands in Arizona and New Mexico. In: McShea, William J.; Healy, William M., eds. Oak forest ecosystems: Ecology and management for wildlife. Baltimore, MD: The Johns Hopkins University Press: 304-316. [43538]
87. Ffolliott, Peter F.; Thorud, David B. 1974. Vegetation for increased water yield in Arizona. Tech. Bull. 215. Tucson, AZ: University of Arizona, Agricultural Experiment Station. 38 p. [4448]
88. Fitzhugh, E. Lee; Moir, William H.; Ludwig, John A.; Ronco, Frank, Jr. 1987. Forest habitat types in the Apache, Gila, and part of the Cibola National Forests, Arizona and New Mexico. Gen. Tech. Rep. RM-145. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 116 p. [4206]
89. Flora of North America Editorial Committee, eds. 2022. Flora of North America north of Mexico, [Online]. Flora of North America Association (Producer). Available: http://www.efloras.org/flora_page.aspx?flora_id=1. [36990]
90. Frank, T. D.; Tweddale, S. A. 2006. The effect of spatial resolution on measurement of vegetation cover in three Mojave Desert shrub communities. Journal of Arid Environments. 67(Supplement): 88-99. [95958]
91. Freeman, C. E.; Dick-Peddie, W. A. 1970. Woody riparian vegetation in the Black and Sacramento Mountain ranges, southern New Mexico. The Southwestern Naturalist. 15(2): 145-164. [6470]
92. Friggens, M., Loehman, R., Holsinger, L., Finch, D. 2014. Vulnerability of riparian obligate species to the interactive effect of fire, climate and hydrological change: Final report. Interagency Agreement #13-IA-11221632-006. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. Available: https://www.fs.usda.gov/rm/pubs_journals/2014/rmrs_2014_friggins_m002.pdf. 213 p. [96213]
93. Friggens, Megan M.; Matthews, Stephen N. 2012. Risk assessment for two bird species in northern Wisconsin. In: Vose, James M.; Peterson, David L.; Patel-Weynand, Toral, eds. Effects of climatic variability and change on forest ecosystems: A comprehensive science synthesis for the U.S. forest sector. PNW-GTR-870. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: 256-258. [87089]
94. Galuszka, Donna M.; Kolb, Thomas E. 2002. Tree growth and regeneration response to climate and stream flow in a species-rich southwestern riparian forest. Western North American Naturalist. 62(3): 266-279. [43101]
95. Garrison, George A.; Bjugstad, Ardell J.; Duncan, Don A.; Lewis, Mont E.; Smith, Dixie R. 1977. Vegetation and environmental features of forest and range ecosystems. Agric. Handb. 475. Washington, DC: U.S. Department of Agriculture, Forest Service. 68 p. [998]
96. Glenn, Edward P.; Nagler, Pamela L. 2005. Comparative ecophysiology of Tamarix ramosissima and native trees in western U.S. riparian zones. Journal of Arid Environments. 61(3): 419-446. [51922]
97. Glinski, Richard L. 1977. Regeneration and distribution of sycamore and cottonwood trees along Sonoita Creek, Santa Cruz County, Arizona. In: Johnson, R. Roy; Jones, Dale A., tech. coords. Importance, preservation and management of riparian habitat: A symposium: Proceedings; 1977 July 9; Tucson, AZ. Gen. Tech. Rep. RM-43. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 116-123. [5340]
98. Gresswell, Robert E. 1999. Fire and aquatic ecosystems in forested biomes of North America. Transactions of the American Fisheries Society. 128(2): 193-221. [31018]
99. Guardia, Merce; Diaz, Raquel; Save, Robert; Aleta, Neus. 2013. Autumn frost resistance on several walnut species: Methods comparison and impact of leaf fall. Forest Science. 59(5): 559-565. [96277]
100. Hall, David H.; Steidl, Robert J. 2007. Movements, activity, and spacing of Sonoran mud turtles (Kinosternon sonoriense) in interrupted mountain streams. Copeia. 2007(2): 403-412. [96241]
101. Hall, Linnea S.; Morrison, Michael L.; Christoferson, Laurel L.; Martin, John; Bock, Carl E.; Strong, Thomas R. 2002. Bird populations in riparian areas of southeastern Arizona in 1985-1986 and 1994-1995. Western North American Naturalist. 62(3): 370-376. [43921]
102. Harlan, Annita; Dennis, Arthur E. 1976. A preliminary plant geography of Canyon de Chelly National Monument. Journal of the Arizona Academy of Science. 11(2): 69-78. [75981]
103. Hermann, Jack A. 1950. The mammals of the Stockton Plateau of northeastern Terrell County, Texas. The Texas Journal of Science. 2(3): 368-393. [61365]
104. Howe, W. H.; Knopf, F. L. 1991. On the imminent decline of Rio Grande cottonwoods in central New Mexico. The Southwestern Naturalist. 36(2): 218-224. [18278]
105. Hultine, K. R.; Cable, W. L.; Burgess, S. S. O.; Williams, D. G. 2003. Hydraulic redistribution by deep roots of a Chihuahuan Desert phreatophyte. Tree Physiology. 23(5): 353-360. [96279]
106. Hultine, K. R.; Williams, D. G.; Burgess, S. S. O.; Keefer, T. O. 2003. Contrasting patterns of hydraulic redistribution in three desert phreatophytes. Oecologia. 135(2): 167-175. [96278]
107. Hunt, Duston Lamar, Jr. 1978. Diet and habitat utilization of tame mule deer in a pinyon-juniper woodland. Las Cruces, NM: New Mexico State University. 82 p. Thesis. [85057]
108. Jemison, Roy. 2004. Relationships between hydrology, exotic plants, and fuel loads in the Middle Rio Grande. In: Hydrology and water resources in Arizona and the Southwest: Proceedings, Arizona-Nevada Academy of Science--Hydrology section; 2003 April 12; Flagstaff, AZ. Volume 33. Glendale, AZ: Arizona Nevada Academy of Science: 85-92. [52856]
109. Johnson, R. Roy; Bennett, Peter S.; Haight, Lois T. 1989. Southwestern woody riparian vegetation and succession: An evolutionary approach. In: Abell, Dana L., technical coordinator. Proceedings of the California riparian systems conference: Protection, management, and restoration for the 1990's; 1988 September 22-24; Davis, CA. Gen. Tech. Rep. PSW-110. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: 135-139. [13515]
110. Johnson, R. Roy; Cartron, Jean-Luc E.; Haight, Lois T.; Duncan, Russell B.; Kingsley, Kenneth J. 2000. A historical perspective on the population decline of the cactus ferruginous pygmy-owl in Arizona. In: Cartron, Jean-Luc E.; Finch, Deborah M., tech. eds. Ecology and conservation of the cactus ferruginous pygmy-owl in Arizona. Gen. Tech. Rep. RMRS-GTR-43. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 17-26. [35490]
111. Johnson, R. Roy; Johnson, Elaine E.; Carothers, Steven W. 2018. Terrestrial vertebrates of mesquite bosque in southwestern North America. In: Johnson, R. Roy; Carothers, Steven W.; Finch, Deborah M.; Kingsley, Kenneth J.; Stanley, John T., tech. eds. Riparian research and management: Past, present, future: Volume 1. Gen. Tech. Rep. RMRS-GTR-377. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 188-211. [93229]
112. Kaib, Mark J. 1998. Fire history in riparian canyon pine-oak forests and the intervening desert grasslands of the Southwest borderlands: A dendroecological, historical, and cultural inquiry. Tucson, AZ: University of Arizona. 234 p. Thesis. [88516]
113. Kaib, Mark; Baisan, Christopher H.; Grissino-Mayer, Henri D.; Swetnam, Thomas W. 1996. Fire history in the gallery pine-oak forests and adjacent grasslands of the Chiricahua Mountains of Arizona. In: Ffolliott, Peter F.; DeBano, Leonard F.; Baker, Malchus B., Jr.; Gottfried, Gerald J.; Solis-Garza, Gilberto; Edminster, Carleton B.; Neary, Daniel G.; Allen, Larry S.; Hamre, R. H., tech. coords. Effects of fire on Madrean Province ecosystems: A symposium proceedings; 1996 March 11-15; Tucson, AZ. Gen. Tech. Rep. RM-GTR-289. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 253-264. [28109]
114. Kartesz, J. T. The Biota of North America Program (BONAP). 2015. North American Plant Atlas, [Online]. Chapel Hill, NC: The Biota of North America Program (Producer). Available: http://bonap.net/napa [maps generated from Kartesz, J. T. 2015. Floristic synthesis of North America, Version 1.0. Biota of North America Program (BONAP)] (in press). [94573]
115. Kartesz, J. T., The Biota of North America Program (BONAP). 2015. Taxonomic Data Center, [Online]. Chapel Hill, NC: The Biota of North America Program (Producer). Available: http://bonap.net/tdc [maps generated from Kartesz, J. T. 2015. Floristic synthesis of North America, Version 1.0. Biota of North America Program (BONAP)] (in press). [84789]
116. Keane, Robert E.; Mincemoyer, Scott A.; Schmidt, Kirsten M.; Long, Donald G.; Garner, Janice L. 2000. Mapping vegetation and fuels for fire management on the Gila National Forest Complex, New Mexico. Gen. Tech. Rep. RMRS-GTR-46. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 126 p. [91538]
117. Kearney, Thomas H.; Peebles, Robert H.; Howell, John Thomas; McClintock, Elizabeth. 1960. Arizona flora. 2nd ed. Berkeley, CA: University of California Press. 1085 p. [6563]
118. Ketcham, Shari L.; Koprowski, John L.; Falk, Donald A. 2017. Differential response of native Arizona gray squirrels and introduced Abert's squirrels to a mosaic of burn severities. Mammal Study. 42(4): 1-12. [95122]
119. Knipe, O. D. 1982. Angora goats for conversion of Arizona chaparral: Early results. In: Conrad, C. Eugene; Oechel, Walter C., technical coordinators. Proceedings of the symposium on dynamics and management of Mediterranean-type ecosystems; 22-26 June 1981; San Diego, CA. Gen. Tech. Rep. PSW-58. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: 264-269. [6028]
120. Koprowski, John L.; Corse, Michelle C. 2001. Food habits of the Chiricahua fox squirrel (Sciurus nayaritensis chiricahuae). Southwestern Naturalist. 46(1): 62-65. [96280]
121. Krochmal, Arnold; Krochmal, Connie. 1982. Uncultivated nuts of the United States. Agriculture Information Bulletin 450. Washington, DC: U.S. Department of Agriculture, Forest Service. 89 p. [1377]
122. Kuchler, A. W. 1964. Manual to accompany the map of potential vegetation of the conterminous United States. Special Publication No. 36. New York: American Geographical Society. 166 p. [1384]
123. Kufeld, Roland C.; Wallmo, O. C.; Feddema, Charles. 1973. Foods of the Rocky Mountain mule deer. Res. Pap. RM-111. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 31 p. [1387]
124. Kurmes, Ernest A.; Wommack, Donald E. 1980. Arizona cypress. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 117 p. [50052]
125. Lambert, Adam M.; D'Antonio, Carla M.; Dudley, Tom L. 2010. Invasive species and fire in California ecosystems. Fremontia. 38(2): 29-36. [89006]
126. LANDFIRE Biophysical Settings. 2009. Biophysical setting 1511552: North American warm desert riparian systems - Stringers. In: LANDFIRE Biophysical Setting Model: Map zone 15, [Online]. Washington, DC: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory; U.S. Geological Survey; Arlington, VA: The Nature Conservancy (Producers). Available: https://www.fs.usda.gov/database/feis/pdfs/BpS/1511552.pdf. [96194]
127. LANDFIRE. 2008. CONUS refresh (LANDFIRE 1.1.0). Biophysical settings layer, LANDFIRE data distribution site, [Online]. In: LANDFIRE. U.S. Department of the Interior, Geological Survey (Producer). Available: https://landfire.cr.usgs.gov/viewer/ [2017, January 10]. [89416]
128. Layser, Earle F.; Schubert, Gilbert H. 1979. Preliminary classification for the coniferous forest and woodland series of Arizona and New Mexico. Res. Pap. RM-208. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 27 p. [1428]
129. Levine, C. M.; Stromberg, J. C. 2001. Effects of flooding on native and exotic plant seedlings: Implications for restoring south-western riparian forests by manipulating water and sediment flows. Journal of Arid Environments. 49(1): 111-131. [43624]
130. List, Rurik; Ceballos, Gerardo; Curtin, Charles; Gogan, Peter J. P.; Pacheco, Jesus; Truett, Joe. 2007. Historic distribution and challenges to bison recovery in the northern Chihuahuan Desert. Conservation Biology. 21(6): 1487-1494. [69217]
131. Lite, S. J.; Stromberg, J. C. 2005. Surface water and ground-water thresholds for maintaining Populus-Salix forests, San Pedro River, Arizona. Biological Conservation. 125(2): 153-167. [53987]
132. Little, Elbert L., Jr. 1950. Southwestern trees: A guide to the native species of New Mexico and Arizona. Agric. Handb. No. 9. Washington, DC: U.S. Department of Agriculture, Forest Service. 109 p. [20317]
133. Little, Elbert L., Jr. 1976. Atlas of United States trees. Volume 3. Minor western hardwoods. Misc. Publ. 1314. Washington, DC: U.S. Department of Agriculture, Forest Service. 13 p. [+ 290 maps]. [10430]
134. Little, Elbert L., Jr. 1979. Checklist of United States trees (native and naturalized). Agric. Handb. 541. Washington, DC: U.S. Department of Agriculture, Forest Service. 375 p. [2952]
135. Lona, Irene D.; Miller, Donald G. III; Hatfield, Colleen A.; Rosecrance, Richard C.; Nelson, Lori J.; Audley, Jackson P.; Siefker, Megan A.; Chen, Yigen; Seybold, Steven J. 2020. Host selection behavior mediated by differential landing rates of the walnut twig beetle, Pityophthorus juglandis, and associated subcortical insect species, on two western North American walnut species, Juglans californica and J. major. Entomologia Experimentalis et Applicata. 168(3): 240-258. [96281]
136. Lowe, Charles H., Jr. 1961. Biotic communities in the sub-Mogollon region of the Inland Southwest. Arizona Academy of Science Journal. 2: 40-49. [20379]
137. Lowe, Charles H. 1964. Arizona's natural environment: Landscapes and habitats. Tucson, AZ: The University of Arizona Press. 136 p. [20736]
138. Magill, Arthur M.; Miller, Carol; Rodgers, Jane E. 2008. Chilopsis linearis (Cov.) Sweet. In: Bonner, Franklin T.; Karrfalt, Robert P., eds. Woody plant seed manual. Agric. Handbook No. 727. Washington, DC: U.S. Department of Agriculture, Forest Service: 396-397. [79134]
139. Manning, Wayne E. 1957. The genus Juglans in Mexico and Central America. Journal of the Arnold Arboretum. 38(2): 121-150. [96282]
140. Marcot, Bruce G. 1995. Owls of old forests of the world. Gen. Tech. Rep. PNW-GRT-343. Portland, OR: U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station. 64 p. [27726]
141. Martin, John A.; Morrison, Michael L. 1999. Distribution, abundance, and habitat characteristics of the buff-breasted flycatcher in Arizona. The Condor. 101(2): 272-281. [36201]
142. Matheron, M. E.; Mircetich, S. M. 1985. Relative resistance of different rootstocks of English walnut to six Phytophthora spp. that cause root and crown rot in orchard trees. Plant Disease. 69(12): 1039-1041. [11544]
143. McClaran, Mitchel P.; Brady, Ward W. 1994. Arizona's diverse vegetation and contributions to plant ecology. Rangelands. 16(5): 208-217. [29721]
144. Medina, Alvin L. 1986. Riparian plant communities of the Fort Bayard watershed in southwestern New Mexico. The Southwestern Naturalist. 31(3): 345-359. [1629]
145. Medina, Alvin L. 1987. Woodland communities and soils of Fort Bayard, southwestern New Mexico. Journal of the Arizona-Nevada Academy of Science. 21(2): 99-112. [3978]
146. Miksicek, Charles H. 1983. Appendix B: Plant remains from agricultural features. In: Teague, Lynn S.; Crown, Patricia L., eds. Hohokam archaeology along the Salt-Gila Aqueduct: Central Arizona Project. Archaeological Series No. 150. Tucson, AZ: Arizona State Museum, Cultural Resource Management Section: 604-620. [Vol. 3: Environment and subsistence; Bureau of Reclamation Contract No. 0-07-32-V0101]. [44341]
147. Miksicek, Charles H. 1984. Historic desertification, prehistoric vegetation change, and Hohokam subsistence in the Salt-Gila Basin. In: Teague, Lynn S.; Crown, Patricia L., eds. Hohokam archaeology along the Salt-Gila Aqueduct: Central Arizona Project. Archaeological Series No. 150. Tucson, AZ: Arizona State Museum, Cultural Resource Management Section: 53-80. [Vol. 7: Environment and subsistence; Bureau of Reclamation Contract No. 0-07-32-V0101]. [44339]
148. Milchunas, Daniel G., ed. 2006. Responses of plant communities to grazing in the southwestern United States. Gen. Tech. Rep. RMRS-GTR-169. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 126 p. [63342]
149. Miller, Regis B. 1976. Reticulate thickenings in some species of Juglans. American Journal of Botany. 63(6): 898-901. [11542]
150. Minckley, W. L.; Brown, David E. 1982. Wetlands. In: Brown, David E., ed. Biotic communities of the American Southwest--United States and Mexico. Desert Plants. 4(1-4): 223-287. [8898]
151. Minckley, W. L.; Clark, Thomas O. 1981. Vegetation of the Gila River Resource Area, eastern Arizona. Desert Plants. 3(3): 124-140. [10863]
152. Muldavin, Esteban H.; DeVelice, Robert L. 1987. A forest habitat type classification of southern Arizona and its relationship to forests of the Sierra Madre Occidental of Mexico. In: Aldon, Earl F.; Gonzales Vicente, Carlos E.; Moir, William H., tech. coords. Strategies for classification and management of native vegetation for food production in arid zones: Proceedings; 1987 October 12-16; Tucson, AZ. Gen. Tech. Rep. RM-150. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 24-31. [2728]
153. Muldavin, Esteban H.; DeVelice, Robert L.; Ronco, Frank, Jr. 1996. A classification of forest habitat types: Southern Arizona and portions of the Colorado Plateau. RM-GTR-287. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 130 p. [27968]
154. Muldavin, Esteban; Durkin, Paula; Bradley, Mike; Stuever, Mary; Mehlhop, Patricia. 2000. Handbook of wetland vegetation communities of New Mexico. Volume 1: Classification and community descriptions. Albuquerque, NM: University of New Mexico, Biology Department; New Mexico Natural Heritage Program. 172 p. [+ appendices]. [45517]
155. Muller, Wolfgang-Ulrich; Leistner, Eckhard. 1978. Aglycones and glycosides of oxygenated naphthalenes and a glycosyltransferase from Juglans. Phytochemistry. 17(10): 1739-1742. [11545]
156. Nagler, Pamela L.; Glenn, Edward P.; Jarnevich, Catherine S. J.; Shafroth, Patrick B. 2011. Distribution and abundance of saltcedar and Russian olive in the western United States. Critical Reviews in Plant Sciences. 30(6): 508–523. [96197]
157. Naiman, Robert J.; Decamps, Henri; Pollock, Michael. 1993. The role of riparian corridors in maintaining regional biodiversity. Ecological Applications. 3(2): 209-212. [96206]
158. NatureServe. 2009. International ecological classification standard: Terrestrial ecological classifications. In: NatureServe Central Databases. Arlington, VA: NatureServe (Producer). 1172 p. Available: http://downloads.natureserve.org/get_data/data_sets/veg_data/nsDescriptions.pdf [2020, August 3]. [94380]
159. NatureServe. 2018. International ecological classification standards: Terrestrial ecological classifications. NVC groups of the eastern U.S. on the LANDFIRE legend. Arlington, VA: NatureServe. 696 p. Available: https://landfire.gov/documents/LANDFIRE_Natural_NVC_Groups_Descriptions_EasternUS.pdf [2021, February 18]. [95146]
160. Nichol, A. A. 1952. [Revised]. The natural vegetation of Arizona. Tech. Bull. 68. Tucson, AZ: University of Arizona, Agricultural Experiment Station: 189-230. [Revisions by W. S. Phillips]. [3928]
161. O'Brien, G. Patrick. 1983. Power pole damage by acorn woodpeckers in southeastern Arizona. In: Davis, Jerry W.; Goodwin, Gregory A.; Ockenfeis, Richard A., technical coordinators. Snag habitat management: Proceedings of the symposium; 1983 June 7-9; Flagstaff, AZ. Gen. Tech. Rep. RM-99. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 14-18. [17813]
162. Ouden, H. Den; Theunissen, J. 1980. Controlled release of naphthalene: A repellent against oviposition of the cabbage root fly, Delia brassicae. Netherlands Journal of Plant Pathology. 86: 17-25. [96032]
163. O’Connor, Christopher D.; Falk, Donald A.; Garfin, Gregg M. 2020. Projected climate-fire interactions drive forest to shrubland transition on an Arizona Sky Island. Frontiers in Environmental Science. 8(137): 1-17. [95923]
164. Papaj, Daniel R. 1994. Oviposition site guarding by male walnut flies and its possible consequences for mating success. Behavioral Ecology and Sociobiology. 34(3): 187-195. [96284]
165. Pase, Charles P.; Layser, Earle F. 1977. Classification of riparian habitat in the Southwest. In: Johnson, Roy; Jones, Dale A., technical coordinators. Importance, preservation and management of riparian habitat: A symposium; 1977 July 9; Tucson, AZ. Gen. Tech. Rep. RM-43. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: 5-9. Available from: NTIS, Springfield, VA 22151; PB-274 582. [5333]
166. Peet, Robert K. 1988. Forests of the Rocky Mountains. In: Barbour, Michael G.; Billings, William Dwight, eds. North American terrestrial vegetation. New York: Cambridge University Press: 63-101. [6714]
167. Peterson, Eric B. 2008. International vegetation classification alliances and associations occurring in Nevada with proposed additions. Carson City, NV: Nevada Natural Heritage Program. 347 p. [77864]
168. Pettit, Neil E.; Naiman, Robert J. 2007. Fire in the riparian zone: Characteristics and ecological consequences. Ecosystems. 10(5): 673-687. [68289]
169. Pope, Dennis P.; Brock, John H.; Backhaus, Ralph A. 1990. Vegetative propagation of key southwestern woody riparian species. Desert Plants. 10(2): 91-95. [11834]
170. Potter, Daniel; Gao, Fangyou; Baggett, Scott; McKenna, James R.; McGranahan, Gale H. 2002. Defining the sources of Paradox: DNA sequence markers for North American walnut (Juglans L.) species and hybrids. Scientia Horticulturae. 94(1-2): 157-170. [96234]
171. Powell, A. Michael. 1988. Trees and shrubs of Trans-Pecos Texas: Including Big Bend and Guadalupe Mountains National Parks. Big Bend National Park, TX: Big Bend Natural History Association. 536 p. [6130]
172. Price, Theresa Lorez. 2008. Flora of Mount Ord, central Arizona and habitat characteristics of Fremontodendron californicum. Tempe, AZ: Arizona State University. 120 p. Thesis. [94820]
173. Racher, Brent J.; Britton, Carlton M. 2003. Fire in saltcedar ecosystems. In: Proceedings: Saltcedar and water resources symposium; 2003 July 16–17; San Angelo, TX. [Publisher name unknown] 94–99. [96196]
174. Raunkiaer, C. 1934. The life forms of plants and statistical plant geography. Oxford, England: Clarendon Press. 632 p. [2843]
175. Read, Ralph A.; Sprackling, John. 1980. Cottonwood-willow--Forest Cover Type 235. In: Eyre, F. H., ed. Forest cover types of the United States and Canada. Washington, DC: Society of American Foresters: 113 p. [50049]
176. Reemts, Charlotte M.; Hansen, Laura L. 2008. Slow recolonization of burned oak-juniper woodlands by Ashe juniper (Juniperus ashei): Ten years of succession after crown fire. Forest Ecology and Management. 255(3-4): 1057-1066. [69954]
177. Reemts, Charlotte. 2012. Management Project Summary: Response of woody species to crown fires in oak-juniper woodlands (Texas). In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: https://www.fs.usda.gov/database/feis/ [2012, December 20]. [86399]
178. Reeves, Timothy. 1976. Vegetation and flora of Chiricahua National Monument, Cochise County, Arizona. Tempe, AZ: Arizona State University. 180 p. Thesis. [20385]
179. Rehder, Alfred. 1940. Manual of cultivated trees and shrubs. New York: MacMillan. 996 p. [21991]
180. Reichhardt, Karen. 1990. Autecology of Arizona sycamore (Platanus wrightii Wats.), a critically important species in south-central Arizona. Tech. Rep. No. 33. Tucson, AZ: University of Arizona, Cooperative National Park Resources Studies Unit; San Francisco, CA: Department of the Interior, National Park Service, Western Region. 13 p. [15363]
181. Reid, M.; Schulz, K.; Schindel, M.; Comer, P.; Kittel, G.; [and others], compilers. 2000. International classification of ecological communities: Terrestrial vegetation of the western United States--Chihuahuan Desert subset. Report from Biological Conservation Datasystem and working draft of April 23, 2000. Boulder, CO: Association for Biodiversity Information/The Nature Conservancy, Community Ecology Group. 154 p. In: Southwestern Regional Gap Analysis Project. Reston, VA: U.S. Geological Survey, Gap Analysis Program (Producer). [52906]
182. Reynolds, Hudson G.; Johnson, R. Roy. 1964. Habitat relations of vertebrates of the Sierra Ancha Experimental Forest. Res. Pap. RM-4. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 16 p. [13485]
183. Reynolds, Lindsay V.; Cooper, David J. 2011. Ecosystem response to removal of exotic riparian shrubs and a transition to upland vegetation. Plant Ecology. 212(8): 1243-1261. [83418]
184. Reynolds, Richard T.; Sanchez Meador, Andrew J.; Youtz, James A.; Nicolet, Tessa; Matonis, Megan S.; Jackson, Patrick L.; DeLorenzo, Donald G.; Graves, Andrew D. 2013. Restoring composition and structure in southwestern frequent-fire forests: A science-based framework for improving ecosystem resiliency. Gen. Tech. Rep. RMRS-GTR-310. U.S. Forest Service, Rocky Mountain Research Station. 76 p. [88928]
185. Rice, Peter M.; McPherson, Guy R.; Rew, Lisa J. 2008. Fire and nonnative invasive plants in the Interior West bioregion. In: Zouhar, Kristin; Smith, Jane Kapler; Sutherland, Steve; Brooks, Matthew L., eds. Wildland fire in ecosystems: Fire and nonnative invasive plants. Gen. Tech. Rep. RMRS-GTR-42-vol. 6. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: 141-173. [70332]
186. Riffle, Jerry W.; Peterson, Glenn W., tech. coords. 1986. Diseases of trees in the Great Plains. Gen. Tech. Rep. RM-129. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station. 149 p. [16990]
187. Ringold, Paul L.; Magee, Teresa K.; Peck, David V. 2008. Twelve invasive plant taxa in US western riparian ecosystems. Journal of the North American Benthological Society. 27(4): 949-966. [73064]
188. Rink, Glenn. 2005. A checklist of the vascular flora of Canyon de Chelly National Monument, Apache County, Arizona. Journal of the Torrey Botanical Society. 132(3): 510-532. [80042]
189. Rodriguez, Elena; Parsons, Jason G.; Peralta-Videa, Jose R.; Cruz-Jimenez, Gustavo; Romero-Gonzalez, Jaime; Sanchez-Salcido, Blanca E.; Saupe, Geoffrey B.; Duarte-Gardea, Maria; Gardea-Torresdey, Jorge L. 2007. Potential of Chilopsis linearis for gold phytomining: Using Xas to determine gold reduction and nanoparticle formation within plant tissues. International Journal of Phytoremediation. 9(2): 133-147. [95980]
190. Rucks, Michael G. 1984. Composition and trend of riparian vegetation on five perennial streams in southeastern Arizona. In: Warner, Richard E.; Hendrix, Kathleen M., eds. California riparian systems: Ecology, conservation, and productive management: Proceedings of a conference; 1981 September 17-19; Davis, CA. Berkeley, CA: University of California Press: 97-107. [5831]
191. Schelz, Charles D.; Scher, Douglas A.; Vegh, Tibor; Abella, Scott R. 2017. How many Arizona walnut trees inhabit Walnut Canyon National Monument? The Southwestern Naturalist. 62(2): 157-161. [96124]
192. Schmutz, E. M.; Smith, E. L.; Ogden, P. R.; Cox, M. L.; Klemmedson, J. O.; Norris, J. J.; Fierro, L. C. 1992. Desert grassland. In: Coupland, R. T., ed. Natural grasslands: Introduction and western hemisphere. Ecosystems of the World 8A. Amsterdam, Netherlands: Elsevier Science Publishers B. V: 337-362. [23832]
193. Seavy, Nathaniel E.; Gardali, Thomas; Golet, Gregory H.; Griggs, F. Thomas; Howell, Christine A.; Kelsey, Rodd; Small, Stacy L.; Viers, Joshua H.; Weigand, James F. 2009. Why climate change makes riparian restoration more important than ever: Recommendations for practice and research. Ecological Restoration. 27(3): 330-338. [96229]
194. Shaw, Harley G. 2006. Wood plenty, grass good, water none: Vegetation changes in Arizona's upper Verde River watershed from 1850 to 1997. Gen. Tech. Rep. RMRS-GTR-177. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 50 p. [65070]
195. Shields, Lora Mangum; Crispin, Joe. 1956. Vascular vegetation of a recent volcanic area in New Mexico. Ecology. 37(2): 341-351. [48580]
196. Shiflet, Thomas N., ed. 1994. Rangeland cover types of the United States. Denver, CO: Society for Range Management. 152 p. [23362]
197. Shreve, Forrest. 1917. The vegetation of a desert mountain range as conditioned by climatic factors. The Journal of Ecology. 5(1): 45-52. [64753]
198. Sidman, Gabriel; Guertin, D. Phillip; Goodrich, David C.; Thoma, David; Falk, Donald; Burns, I. Shea. 2016. A coupled modelling approach to assess the effect of fuel treatments on post-wildfire runoff and erosion. International Journal of Wildland Fire. 25(3): 351-362. [96201]
199. Simpson, Benny J. 1988. A field guide to Texas trees. Austin, TX: Texas Monthly Press. 372 p. [11708]
200. Skartvedt, Peter H. 2000. Woody riparian vegetation patterns in the upper Mimbres Watershed, southwestern New Mexico. The Southwestern Naturalist. 45(1): 6-14. [38910]
201. Smith, D. Max; Finch, Deborah M. 2016. Riparian trees and aridland streams of the southwestern United States: An assessment of the past, present, and future. Journal of Arid Environments. 135(23): 120-131. [96185]
202. Smith, D. Max; Finch, Deborah M. 2017. Climate change and wildfire effects in aridland riparian ecosystems: An examination of current and future conditions. Gen. Tech. Rep. RMRS-GTR-364. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 65 p. [96256]
203. Smith, D. Max; Finch, Deborah M.; Gunning, Christian; Jamison, Roy; Kelly, Jeffrey F. 2009. Post-wildfire recovery of riparian vegetation during a period of water scarcity in the southwestern USA. Fire Ecology--Special Issue. 5(1): 38-55. [77004]
204. Smith, G. Shannon; Pittcock, Kim. 1989. The collector's quest. American Nurseryman. 169(1): 56-65. [12243]
205. Snyder, Keirith A.; Guertin, D. Phillip; Jemison, Roy L.; Ffolliott, Peter F. 2002. Riparian plant community patterns: A case study from southeastern Arizona. Journal of the Arizona-Nevada Academy of Science. 34(2): 106-111. [45198]
206. Song, Xiao-Bo; Ma, Qing-Guo; Zhou, Ye; Chang, Ying-Ying; Zhang, Jun-Pei; Pei, Dong. 2020. The complete chloroplast genome of paradox (Juglans major x J. regia), an interspecific hybrid in China. Mitochondrial DNA Part B. 5(3): 2087-2088. [96286]
207. Stambaugh, Michael C.; Guyette, Richard P.; Godfrey, Ralph; McMurry, E. R.; Marschall, J. M. 2009. Fire, drought, and human history near the western terminus of the Cross Timbers, Wichita Mountains, Oklahoma, USA. Fire Ecology. 5(2): 51-65. [81033]
208. Steele, Robert. 1988. Ecological relationships of ponderosa pine. In: Baumgartner, David M.; Lotan, James E., comps. Ponderosa pine: The species and its management: Symposium proceedings; 1987 September 29 - October 1; Spokane, WA. Pullman, WA: Washington State University, Cooperative Extension: 71-76. [9402]
209. Stevens, Larry E. 2002. Exotic tamarisk on the Colorado Plateau, [Online]. In: Grahame, John D.; Sisk, Thomas D., eds. Canyons, cultures and environmental change: An introduction to the land-use history of the Colorado Plateau. U.S. Geological Survey (Producer). Available: https://web.archive.org/web/20060206062038/http://www.cpluhna.nau.edu/Biota/tamarisk.htm [2021, December 15, 2021]. [44465]
210. Stickney, Peter F. 1989. Seral origin of species comprising secondary plant succession in northern Rocky Mountain forests. FEIS workshop: Postfire regeneration. Unpublished draft on file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Missoula Fire Sciences Laboratory. 10 p. [20090]
211. Stoleson, Scott H.; Finch, Deborah M. 1999. Unusual nest sites for southwestern willow flycatchers. Wilson Bulletin. 111(4): 574-575. [41441]
212. Stromberg, J. C. 1993. Fremont cottonwood-Goodding willow riparian forests: A review of their ecology, threats, and recovery potential. Journal of the Arizona-Nevada Academy of Sciences. 27(1): 97-110. [29724]
213. Stromberg, J. C. 2001. Biotic integrity of Platanus wrightii riparian forests in Arizona: First approximation. Forest Ecology and Management. 142(1-3): 251-266. [96257]
214. Stromberg, J. C.; McCluney, K. E.; Dixon, M. D.; Meixner, T. 2013. Dryland riparian ecosystems in the American Southwest: Sensitivity and resilience to climatic extremes. Ecosystems. 16(3): 411-415. [96205]
215. Stromberg, J. C.; Tiller, R.; Richter, B. 1996. Effects of groundwater decline on riparian vegetation of semiarid regions: The San Pedro, Arizona. Ecological Applications. 6(1): 113-131. [48991]
216. Stromberg, Juliet C. 1998. Functional equivalency of saltcedar (Tamarix chinensis) and Fremont cottonwood (Populus fremontii) along a free-flowing river. Wetlands. 18(4): 675-686. [43989]
217. Stromberg, Juliet C.; Patten, Duncan T. 1990. Flower production and floral ratios of a southwestern riparian tree, Arizona walnut (Juglans major). The American Midland Naturalist. 124(2): 278-288. [13780]
218. Stromberg, Juliet C.; Patten, Duncan T. 1990. Seed production and seedling establishment of a southwest riparian tree, Arizona walnut (Juglans major). The Great Basin Naturalist. 50(1): 47-56. [11169]
219. Stromberg, Juliet C.; Patten, Duncan T. 1990. Variation in seed size of a southwestern riparian tree, Arizona walnut (Juglans major). The American Midland Naturalist. 124(2): 269-277. [13781]
220. Stromberg, Juliet C.; Rychener, Tyler J. 2010. Effects of fire on riparian forests along a free-flowing dryland river. Wetlands. 30(1): 75-86. [81928]
221. Strong, Thomas R., Bock, Carl E. 1990. Bird species distribution patterns in riparian habitats in southeastern Arizona. The Condor. 92(4): 866-885. [69088]
222. Stuever, Mary C. 1997. Fire-induced mortality of Rio Grande cottonwood. Albuquerque, NM: University of New Mexico. 85 p. Thesis. [44706]
223. Stuever, Mary C.; Hayden, John S. 1996. Plant associations (habitat types) of the forests and woodlands of Arizona and New Mexico. Final report: Contract R3-95-27. Placitas, NM: Seldom Seen Expeditions. 520 p. [28868]
224. Swadek, Rebecca K.; Burgess, Tony L. 2012. The vascular flora of the north central Texas Walnut Formation. Journal of the Botanical Research Institute of Texas. 6(2): 725-752. [96289]
225. Szaro, Robert C. 1980. Factors influencing bird populations in southwestern riparian forests. In: DeGraaf, Richard M., technical coordinator. Workshop proceedings: Management of western forests and grasslands for nongame birds; 1980 February 11-14; Salt Lake City, UT. Gen. Tech. Rep. INT-86. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: 403-418. [17916]
226. Szaro, Robert C. 1989. Riparian forest and scrubland community types of Arizona and New Mexico. Desert Plants. 9(3-4): 70-138. [604]
227. Szaro, Robert C. 1990. Southwestern riparian plant communities: Site characteristics, tree species distributions, and size-class structures. Forest Ecology and Management. 33/34: 315-334. [10031]
228. Szaro, Robert C.; King, Rudy M. 1990. Sampling intensity and species richness: Effects on delineating Southwestern riparian plant communities. Forest Ecology and Management. 33/34: 335-349. [13783]
229. Szaro, Robert C.; Pase, Charles P. 1983. Short-term changes in a cottonwood-ash-willow association on a grazed and an ungrazed portion of Little Ash Creek in central Arizona. Journal of Range Management. 36(3): 382-384. [6520]
230. Thomas, Jonathan A. 2015. Tree species influence woodland canopy characteristics and crown fire potential. Forest Ecology and Management. 362: 169-176. [90385]
231. Thomas, Jonathan A.; White, Joseph D.; Murray, Darrel B. 2016. Tree species influence woodland canopy characteristics and crown fire potential. Forest Ecology and Management. 362: 169-176. [94522]
232. Thompson, Robert S.; Anderson, Katherine H.; Bartlein, Patrick J. 1999. Digital representations of tree species range maps from "Atlas of United States trees" by Elbert L. Little, Jr. (and other publications). In: Atlas of relations between climatic parameters and distributions of important trees and shrubs in North America. Denver, CO: U.S. Geological Survey, Information Services (Producer). On file at: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory, Missoula, MT; FEIS files. [92575]
233. Turner, Raymond M.; Bowers, Janice E.; Burgess, Tony L. 2005. Sonoran Desert plants: An ecological atlas. Tucson, AZ: The University of Arizona Press. 504 p. [94363]
234. Tye, Simon P.; Geluso, Keith. 2019. Day roosts of Myotis (Mammalia: Chiroptera) in an arid riparian corridor in southwestern New Mexico. Western North American Naturalist. 79(4): 515-522. [96290]
235. U.S. Fish and Wildlife Service. 2022. Listed species believed or known to occur in New Mexico, [Online]. Environmental Conservation Online System. Available: https://ecos.fws.gov/ecp0/reports/species-listed-by-state-report?state=NM [2022, January 2]. [96188]
236. USDA, Natural Resources Conservation Service. 1997. Riparian forest buffer. Conservation Practice Job Sheet 391. U.S. Department of Agriculture, Natural Resources Conservation Service. 4 p. [96191]
237. USDA, NRCS. 2021. The PLANTS Database, [Online]. Greensboro, NC: U.S. Department of Agriculture, Natural Resources Conservation Service, National Plant Data Team (Producer). Available: https://plants.usda.gov/. [34262]
238. USDI, Fish and Wildlife Service. 2022. Endangered Species Program, [Online]. U.S. Department of the Interior, Fish and Wildlife Service (Producer). Available: https://www.fws.gov/endangered/. [86564]
239. Van Dersal, William R. 1938. Native woody plants of the United States, their erosion-control and wildlife values. Misc. Publ. No. 303. Washington, DC: U.S. Department of Agriculture. 362 p. [4240]
240. van Riper, Charles III; Paxton, Kristina L.; O’Brien, Chris; Shafroth, Patrick B.; McGrath, Laura J. 2008. Rethinking avian response to Tamarix on the Lower Colorado River: A threshold hypothesis. Restoration Ecology. 16(1): 155 167. [96203]
241. Vines, Robert A. 1960. Trees, shrubs, and woody vines of the Southwest. Austin, TX: University of Texas Press. 1104 p. [7707]
242. Walker, Lawrence R.; Barnes, Pamela L.; Powell, Elizabeth A. 2006. Tamarix aphylla: A newly invasive tree in southern Nevada. Western North American Naturalist. 66(2): 191-201. [64514]
243. Wallace, Eric J.; Steidl, Robert J.; Swann, Don E. 2010. Habitat characteristics of lowland leopard frogs in mountain canyons of southeastern Arizona. Journal of Wildlife Management. 74(4): 808-815. [96291]
244. Wallmo, O. C. 1955. Vegetation of the Huachuca Mountains, Arizona. The American Midland Naturalist. 54(2): 466-480. [20325]
245. Walters, M. Alice; Teskey, Robert O.; Hinckley, Thomas M. 1980. Impact of water level changes on woody riparian and wetland communities. Volume 7: Mediterranean region, western arid and semi-arid region. FWS/OBS-78/93. Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service, Biological Services Program. 84 p. [52899]
246. Walters, R. S.; Auchmoody, L. R. 1989. Vegetation re-establishment on a hardwood forest site denuded by brine. Landscape and Urban Planning. 17(2): 127-133. [9819]
247. Warren, Peter L.; Hoy, Marina S.; Hoy, Wilton E. 1992. Vegetation and flora of Fort Bowie National Historic Site, Arizona. Tech. Rep. NPS/WRUA/NRTR-92/43. Tucson, AZ: The University of Arizona, School of Renewable Natural Resources, Cooperative National Park Resources Studies Unit. 78 p. [19871]
248. Wasser, Clinton H. 1982. Ecology and culture of selected species useful in revegetating disturbed lands in the West. FWS/OBS-82/56. Washington, DC: U.S. Department of the Interior, Fish and Wildlife Service, Office of Biological Services, Western Energy and Land Use Team. 347 p. Available from NTIS, Springfield, VA 22161; PB-83-167023. [2458]
249. Wauer, Roland H. 1971. Ecological distribution of birds of the Chisos Mountains, Texas. The Southwestern Naturalist. 16(1): 1-29. [24969]
250. Weakley, Alan S. 2020. Flora of the southeastern United States: Eastern Texas, [Online]. Chapel Hill, NC: University of North Carolina (Producer). Available: http://herbarium.bio.unc.edu/FSUS_2020/FSUS_Eastern_Texas.pdf. [94920]
251. Webb, Amanda D. 2017. Fire effects and management in riparian ecosystems of the southwestern United States and Mexico. Tucson, AZ: University of Arizona. 168 p. Thesis. [92607]
252. Webb, Amanda D.; Falk, Donald A.; Finch, Deborah M. 2019. Fire ecology and management in lowland riparian ecosystems of the southwestern United States and northern Mexico. Gen. Tech. Rep. RMRS-GTR-401. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 132 p. [95328]
253. Webb, Robert H.; Griffiths, Peter G.; Wallace, Cynthia S. A.; Boyer, Diane E. 2007. Channel response to low-elevation desert fire: The King Valley Fire of 2005. Rep. Series Data–DS 275. Tucson, AZ: U.S. Department of the Interior. U.S. Geological Survey. 52 p. [95986]
254. Welsh, Stanley L.; Atwood, N. Duane; Goodrich, Sherel; Higgins, Larry C., eds. 2015. A Utah flora. 5th ed. Provo, UT: Brigham Young University. 987 p. [94185]
255. Wentworth, Thomas R. 1982. Vegetation and flora of the Mule Mountains, Cochise County, Arizona. Journal of the Arizona-Nevada Academy of Science. 17(2/3): 29-44. [82638]
256. Wentworth, Thomas R. 1985. Vegetation on limestone in the Huachuca Mountains, Arizona. The Southwestern Naturalist. 30(3): 385-395. [68682]
257. Whittaker, R. H.; Niering, W. A. 1968. Vegetation of the Santa Catalina Mountains, Arizona: IV. Limestone and acid soils. The Journal of Ecology. 56(2): 523-544. [49020]
258. Wiesenborn, William D. 2020. Phenology and description of a new species of Hoplothrips (Thysanoptera: Phlaeothripidae) in Mojave Desert leaf litter. The Pan-Pacific Entomologist. 96(1): 21-30. [95988]
259. Zouhar, Kris. 2003. Tamarix spp. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: https://www.fs.usda.gov/database/feis/plants/tree/tamspp/all.html. [96488]
260. Zouhar, Kris. 2005. Elaeagnus angustifolia. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: https://www.https://www.fs.usda.gov/database/feis/plants/tree/elaang/all.html. [96187]
261. Zouhar, Kristin; Smith, Jane Kapler; Sutherland, Steve; Brooks, Matthew L. 2008. Wildland fire in ecosystems: Fire and nonnative invasive plants. Gen. Tech. Rep. RMRS-GTR-42-vol. 6. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 355 p. [70897]